Innovative solutions to restore shape and function
Trauma Products
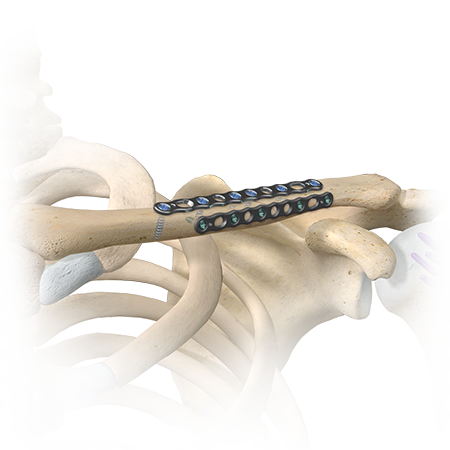
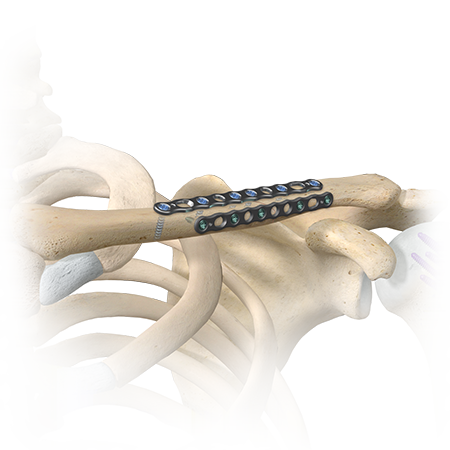
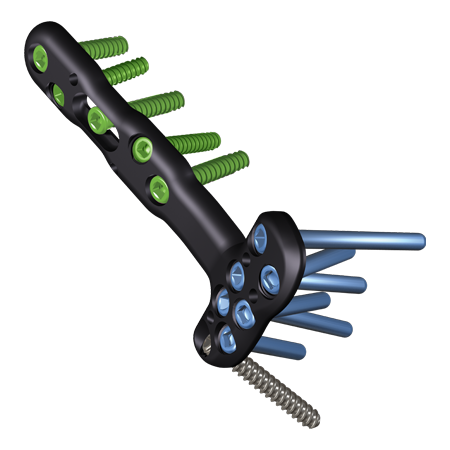
Shoulder & Elbow
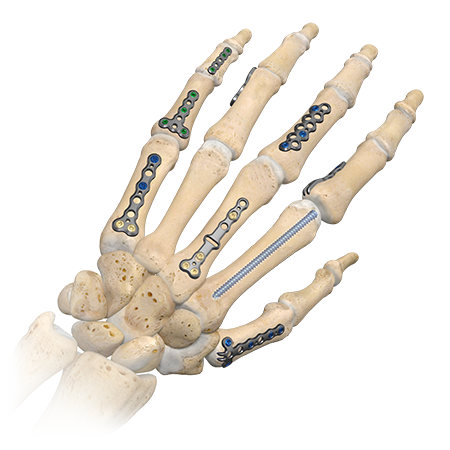
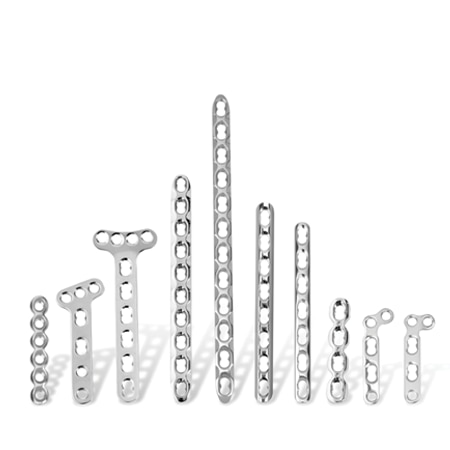
Hand and Wrist
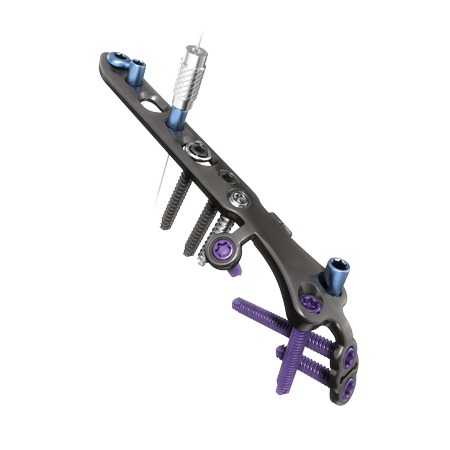
Pelvic
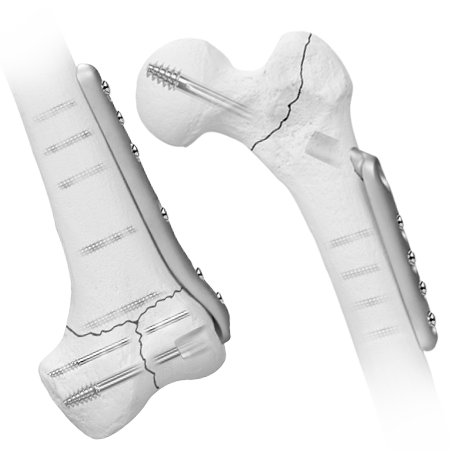
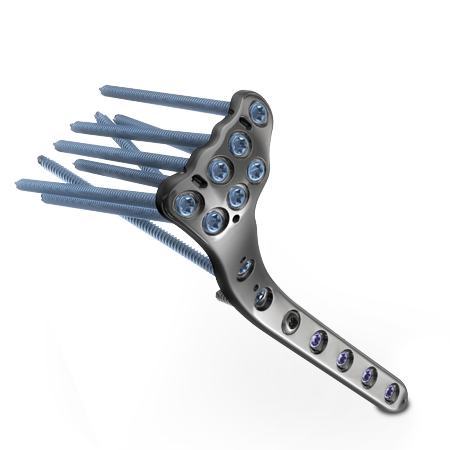
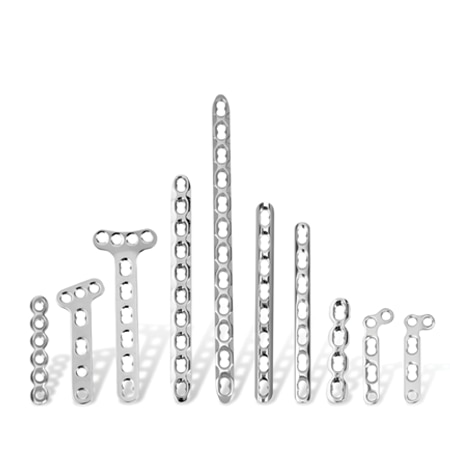
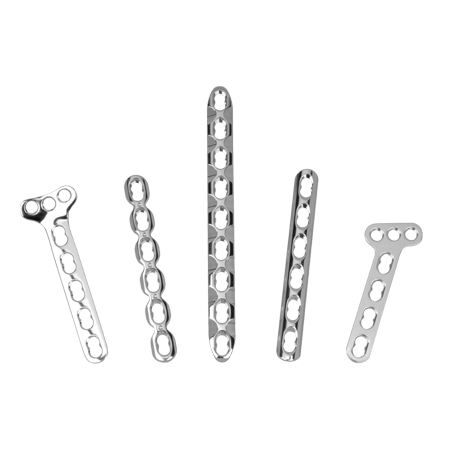
To meet the challenge of treating patients with traumatic injuries, Zimmer Biomet offers a wide range of fracture fixation products specifically designed to address the intricate anatomy and physiology of the extremities.
Hip and Femur
Tibia
Tailored resources for your patients
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.

All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.