Distinctly Designed to Fit the Distal Fibula
A.L.P.S.® Fibula Plating Systems
Combing strength with a low profile
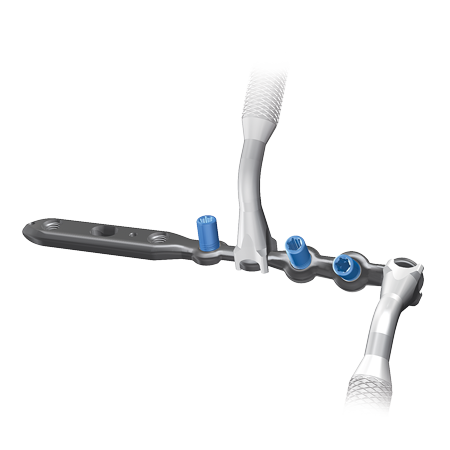
The A.L.P.S. Fibula Plating System aims to make it ideal for simple or complex fibula fracture procedures that involve minimal tissue coverage. The low-profile titanium plate metallurgy and multiplanar locking screw technology enable the formation of the three-dimensional matrix of fixed and variable angle screws to create a true subchondral scaffold that can provide strong fixation in comminuted fractures or osteoporotic bone.
Anatomy
- Ankle
Procedure
- Ankle: Ankle Fracture
System Features
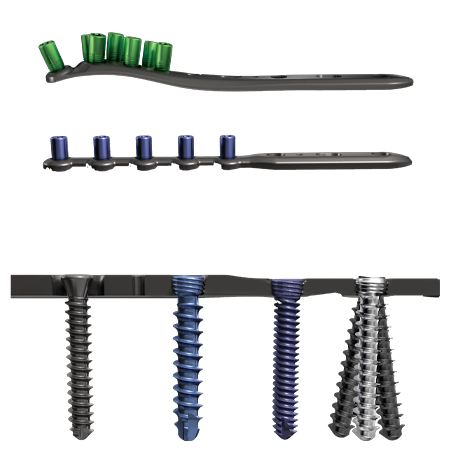
Specifications
Benefits
Unique plate design
- The low profile, anatomic plate mimics anatomy is designed to make it ideal for simple or complex fibula fracture procedures that involve minimal tissue coverage. Composite plate combines the benefits of a locking compression plate with flexible plating technology.
Stability
- The system allows the use of locking, multi-directional, and standard screws. This hybrid fixation concept is designed to allow the surgeon to stabilize the fracture either by the use of lag screw techniques through the plate, or by compression plating techniques. Locking screws serve to provide stability to comminuted, unstable metaphyseal fractures or in osteopenic bone.
Education
Literature
Surgical Approach: The A.L.P.S. Fibula plates feature Type 2 Anodized Titanium Alloy low profile, anatomically contoured implants. In distal fibula surgery where soft tissue coverage is at risk, these low profile plates are designed to minimize discomfort and soft tissue irritation matching the anatomy of the distal fibula, while still having the required strength.
Videos
A.L.P.S. Distal Tibia and Fibula Plating System
Log-in or Register
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.