Zimmer Biomet offers surgeons total knee systems, partial knee systems, bicruciate preserving arthroplasty systems and revision knee systems that empower you to offer a patient specific approach for each knee replacement procedure.
Knee Replacement Products
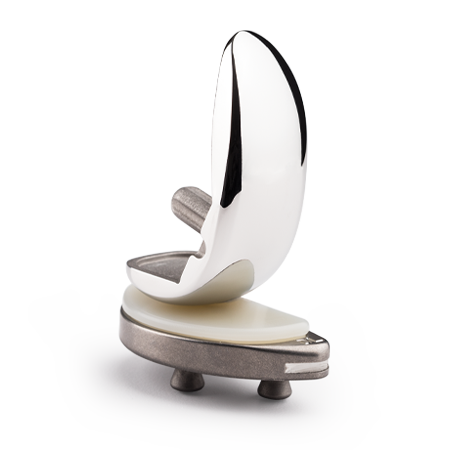
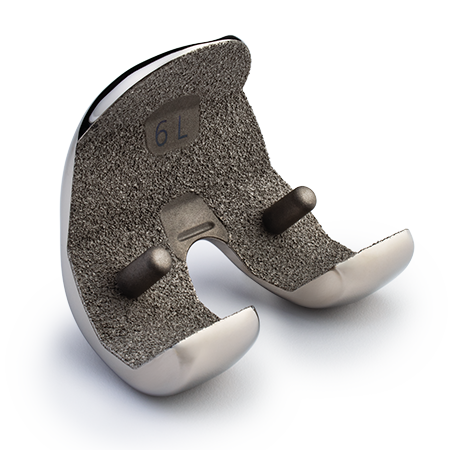
Partial Knee Replacement
Zimmer Biomet is the leading company in partial knee arthroplasty (PKA) with over 45 years of experience, offering a comprehensive range of anatomic and innovative solutions.
Partial Knee Systems
The Oxford Partial Knee is intended for use in individuals with osteoarthritis or avascular necrosis limited to the medial compartment of the knee and is intended to be implanted with bone cement. The Oxford Partial Knee is not indicated for use in the lateral compartment or for patients with ligament deficiency. Potential risks include, but are not limited to, loosening, dislocation, fracture, wear and infection, any of which can require additional surgery.
Primary Knee Replacement
Zimmer Biomet offers surgeons Total Knee Systems that are designed with natural knee motion in mind and offer extensive implant options and combinations, allowing a patient specific approach to reconstruct the knee as individual anatomy dictates.
Primary Knee Systems
Revision Knee Replacement
Zimmer Biomet offers a wide variety of solutions that address fixation, constraint, soft tissue and boney defect management challenges that are often encountered during revision knee arthroplasty.
Revision Knee Systems
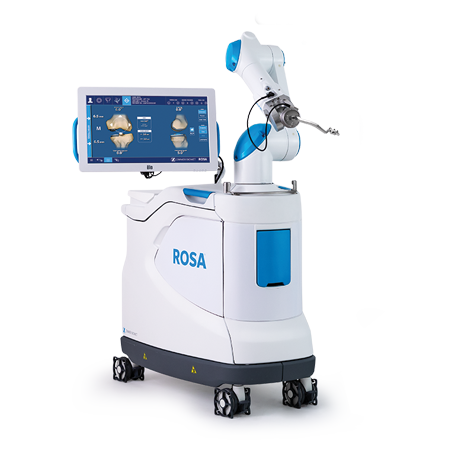
Robotics
The ROSA® Knee System supports surgeons in performing Total Knee Arthroplasty (TKA) with features to assist with the bone resections, as well as assessing the state of the soft tissues to facilitate implant positioning intraoperatively.
Tailored resources for your patients
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.

All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.