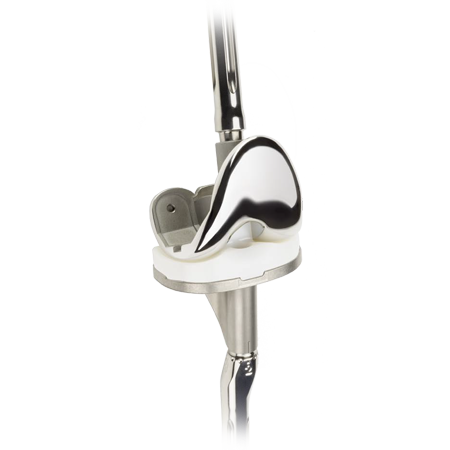
The next generation of rotating hinge knees
NexGen® Rotating Hinge Knee
Bone Conserving, Modular Hinge
The NexGen RH Knee features a bone conserving modular hinge design that addresses key issues related to many conventional rotating hinge knee designs.
Procedures
- Primary Knee Reconstruction
- Revision Knee Reconstruction
Philosophies
- Bone Conservation
- Modularity
Application
- Primary
- Revision
The features of the NexGen RH Knee are designed to allow bone conservation as well as easy intra-operative conversions from NexGen Legacy® Constrained Condylar Knee (LCCK).
Clinical Use
19 yearsThe NexGen Rotating Hinge Knee has over 19 years of clinical use.1
Tibial Condyle Load Bearing
95%The NexGen RH Knee femoral component and articular surfaces are designed to maintain centralized contact throughout ROM (from -3° of hyperextension to 120°) resulting in 95% condylar loading through the tibial condyles.
System Features
Specifications
Benefits
Bone Conserving Hinge Design
- Same femoral cuts as NexGen Legacy Constrained Condylar Knee (LCCK)
Trabecular Metal Tibial and Femoral Cones*
- Trabecular Metal Tibial and Femoral Cones, made from clinically proven2-4 Trabecular Metal Technology, are available in multiple shapes and sizes
*The Zimmer Biomet NexGen Trabecular Metal Tibial and Femoral Cones are for use only with the NexGen Complete Knee Solution System. When used with the RH Knee System, the Trabecular Metal Cones are for cemented use only in the United States. When used with the LCCK System, the Trabecular Metal Cones are for cementless or cemented use.


Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.