Oxford® Cementless
Partial Knee
PROVEN1-3 PREFERRED4* EFFICIENT5,6



The proven 1-3 Oxford Cementless Partial Knee, part of the most preferred 4* partial knee system in the world, allows surgeons to be more efficient 5,6 in the O.R. and helps retain healthy anatomy in patients with Anteromedial Osteoarthritis (AMOA) giving them the best opportunity to get back to what they loved before knee pain7,8.
94%1-3
survivorship at 10 years in the UK National Joint Registry and over 300,000 implanted worldwide9
50%4*
global market share as the
most preferred partial knee implant in the world
≤15 mins5,6
reduce average operating time by up to 15 minutes with the efficient cementless technique
Important Safety Information:
The Oxford® Cementless Partial Knee System is indicated for use in unilateral knee procedures with osteoarthritis or avascular necrosis limited to the medial compartment of the knee. It is intended to be implanted without the application of bone cement for patients whose clinical condition would benefit from a shorter surgical time compared to the cemented implant. The Oxford Partial Knee Is not indicated for use in the lateral compartment or for patients with ligament deficiency, or for use in simultaneous bilateral surgery or planned staged bilateral procedures. Potential risks include, but are not limited to, loosening, dislocation, fracture, wear and infection, any of which can require additional surgery. For a full list of product indications, contraindications and warnings, please see the associated product IFU.
System Features
Patient Preferred.
For patients with AMOA the Oxford Cementless Partial Knee Implant retains healthy anatomy and provides long term fixation3,21 in active patients allowing them the opportunity to get back to what they loved before knee pain 7,8
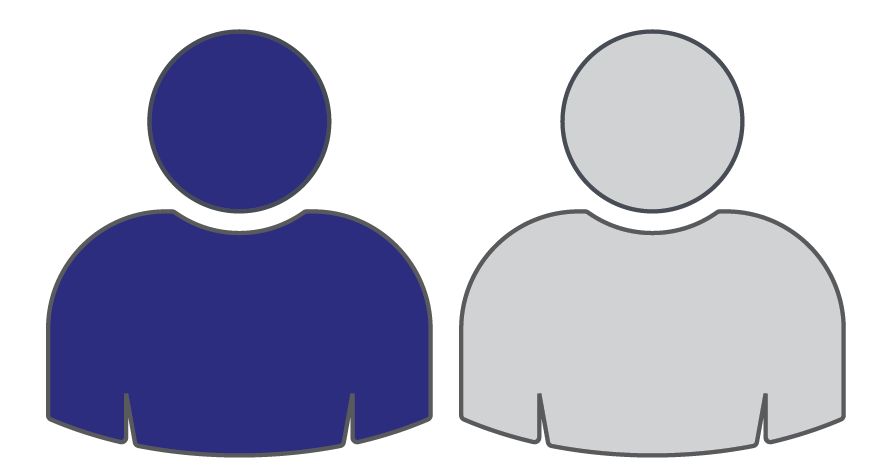
Roughly 1 in 2 patients is a suitable candidate for a PKR22
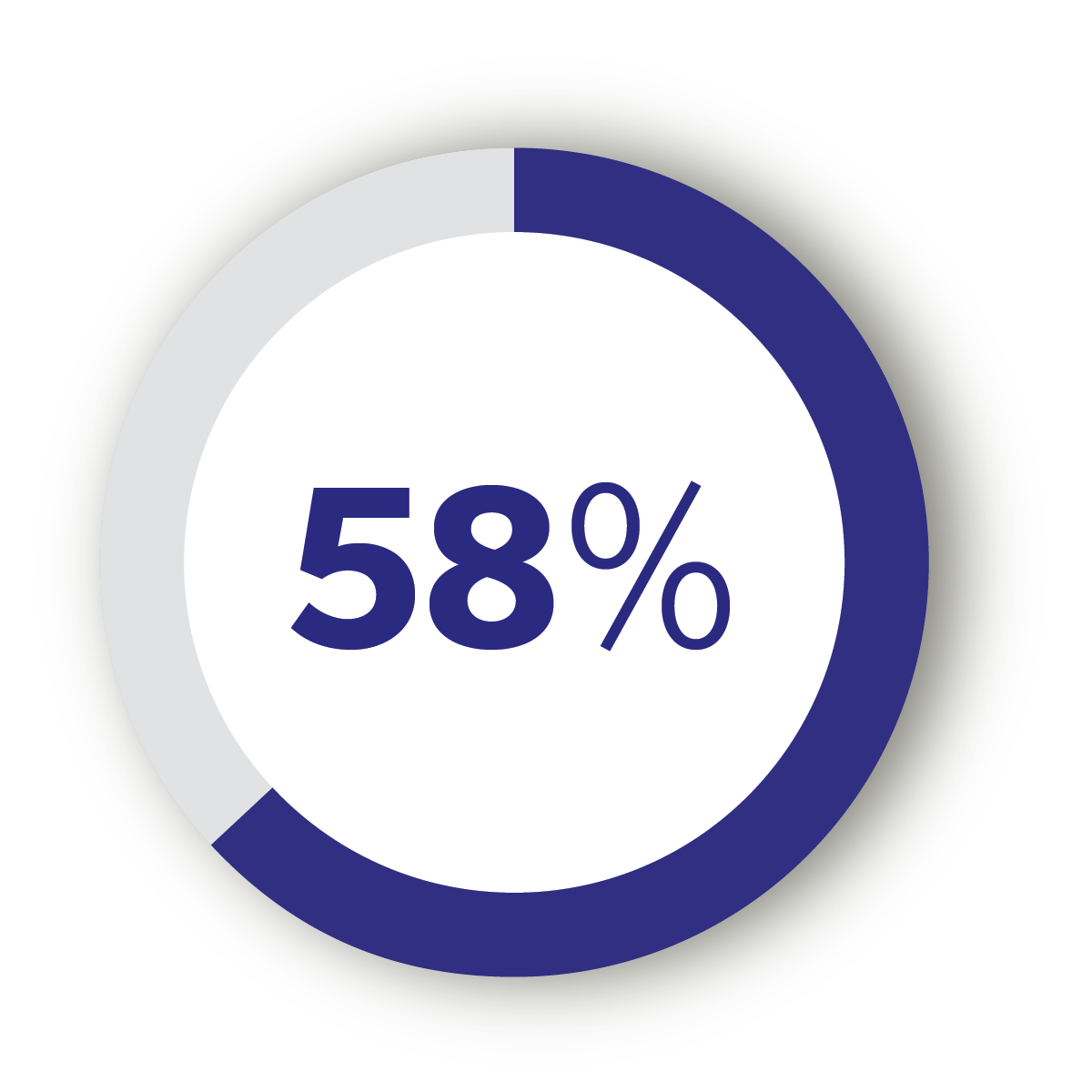
58% of patients would choose a partial knee implant over a total when presented with the various risks and benefits of both options.23
The Industry’s only Lifetime Knee Implant Replacement Warranty in the U.S.†
It’s your assurance that Zimmer Biomet not only makes a proven partial knee, we stand behind it 100%.
What's covered?
Applies to Oxford Partial Knees implanted on or after 4-29-2013
Limited to no more than one complete replacement of the product
Covers the replacement of Oxford Partial Knee components for any reason
Any additional costs associated with surgery or follow-up are not covered – only the implant component
Covers the cost of the replacement implant only; does not cover hospital costs, co-pays, or other related expenses
Brochures and Resources
Videos
Oxford Medical Education Course Video
Sign up to get notified about Oxford Cementless FDA Certified Training in the U.S.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.