Designed to address the unique challenges of proximal humerus fixation.
A.L.P.S® Proximal Humerus Plating System
Intraoperative flexibility and efficiency
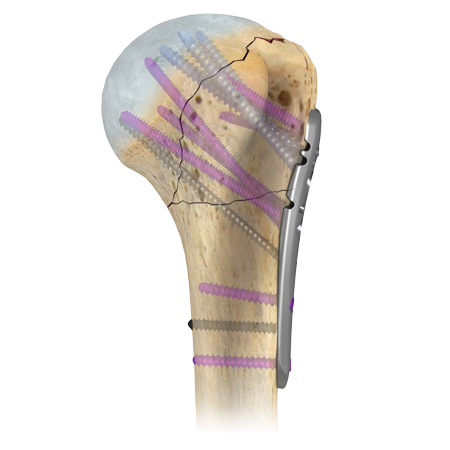
The A.L.P.S. Proximal Humerus Plating System is an integral part of the Zimmer Biomet continuum of care for shoulder treatment. Zimmer Biomet offers a diverse portfolio of options for the life-cycle of patients, from sports-related injuries to fracture fixation to shoulder replacement. It features the next generation in humeral plating, offering the surgeon two plating options based on preference and fracture pattern and TiMAX® surface treatment for strength1 and biocompatibility.
Anatomy
- Shoulder and Elbow
Product Type
- Plates and Screws
System Features
Benefits
Contourable Plates
- Using the ‘feet’ of the benders, the plates can be contoured to conform to the patient’s unique anatomic needs. Pre-contoured anterior plate curvature is designed for insertion, to reduce the possibility that a deltoid release will be necessary.
Implant Stability
- Using the medial calcar screw provides additional stability in the inferior medial cortex. Pegs with blunt tips minimize screw penetration of the articular surface.
Education
Literature
Surgical Approach:
This system offers an extensive selection of anatomically contoured plates, which are available in a variety of lengths and in two styles to accommodate surgeon preference for either high or low application on the greater tuberosity. Long plates curve anteriorly around the deltoid insertion, and can be further contoured in situ for better anatomic fit.
Videos
ALPS® Proximal Humerus Plating System
ALPS® PHP High Plate
ALPS® PHP Low Plate
A.L.P.S. PHP Intro Video
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.