- Head Width: 34mm
- Head Thickness: 3.7mm
- Shaft Width: 11mm
- Shaft Thickness: 3.7mm
- Distance between center holes of shaft: 13mm
- Orientations: Left/Right
- Sizes (holes): 3, 5, 7, 9, 11, 13, 15
- Length (mm): 70.5, 96, 122, 148, 174, 200, 226
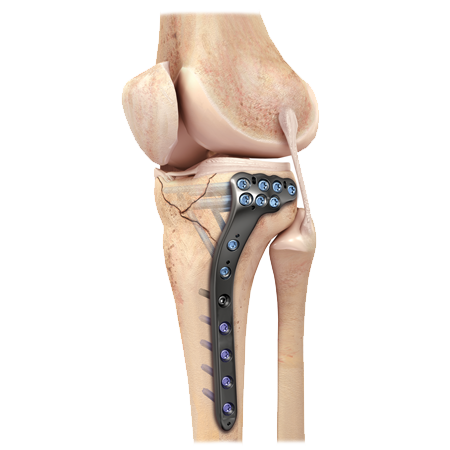
A.L.P.S® Proximal Tibia Plating System
Providing optimal plate conformity and subchondral support
The A.L.P.S. Proximal Tibia plates represent the next generation in anatomic locked plating of tibial plateau fractures. It combines the benefits of low profile titanium plate metallurgy with the advantages of double row, multi-planar locked subchondral screw technology. These features allow the formation of a three-dimensional matrix of fixed and variable angle screws beneath the articular surface to create a true subchondral scaffold that is designed to provide strong fixation in both comminuted fractures and osteopenic bone.
Anatomy
- Tibia
Procedure Type
- Plates and Screws
System Features
Specifications
Proximal Tibia


Benefits
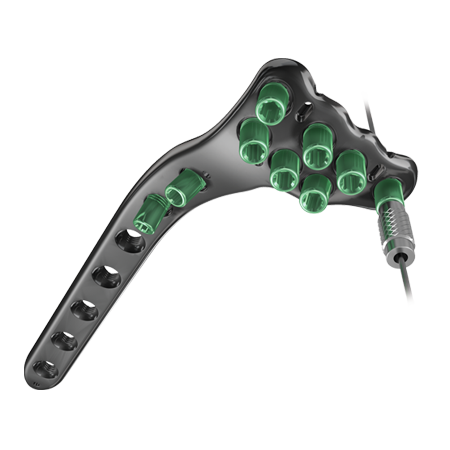
Optimum Plate Conformity
- The A.L.P.S. Proximal Tibia Plates are specifically designed to match the anatomy of the proximal tibia while still having the required strength. This is achieved by offering two distinct lateral proximal tibia plate shapes. The distinction lies with the shape of the slope between the head and the shaft of the plate at the metaphyseal flare. These two plates are categorized as the standard and large curve plates both available in 3, 5, 7, 9, 11, 13 and 15 hole plates.
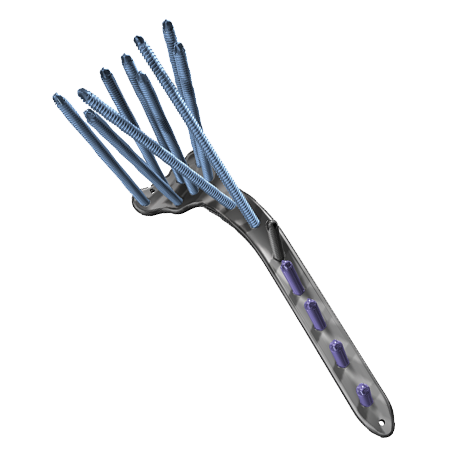
Strength and Support
- Diverging raft row screws and dual kickstand screws offer optimum subchondral support. Dual Raft rows allow sequential stabilization among multiple planes engineered from TiMAX® for strength, biocompatibility and enhanced imaging capabilities over stainless steel.




Videos
ALPS® Proximal Tibia Plating System
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.