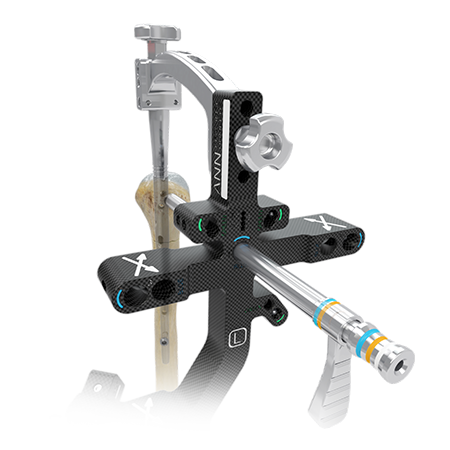
Implants designed to treat a range of humeral fractures.
AFFIXUS® Natural Nail® Humeral System
Efficient. Extensive. Advanced.
The AFFIXUS Natural Nail Humeral System is a long bone nailing system that is built on the Natural Nail and AFFIXUS intramedullary platforms.
Anatomy
- Shoulder and Elbow
Product Type
- Intramedullary Nailing
System Features
Specifications
Benefits
Efficient
- The instrument cases are designed in a simple, step-wise, color-coded layout for ease of use and reproducible procedures for the surgeon and OR staff teams. Instrumentation cases are also designed modularly to provide space and inventory efficiencies to the hospital. Cases include intraoperative options including entry portals, reduction tools, and color-coded screw instrumentation placement.
Extensive
- Created with acute fixation options for many challenging fracture patterns, the AFFIXUS Natural Nail System was designed with two implant options for humeral fractures in both antegrade and retrograde surgical approaches.
Advanced
- The AFFIXUS Natural Nail Proximal Humeral Implant showcases the proprietary CoreLockTM technology, integrating a fixed-angle interlocking mechanism into the nail, allowing the user to lock all metaphyseal screws in the construct at a fixed angle at once.
Education
Literature
Surgical Approach:
The AFFIXUS Natural Nail Humeral System is a long bone nailing system built on the Natural Nail and AFFIXUS intramedullary platforms. This system offers a complete portfolio of implants and instruments, which treats a wide range of humeral fractures using simple and efficient instrumentation.
Videos
AFFIXUS Natural Nail Proximal Humerus Animation
Watch animation
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.