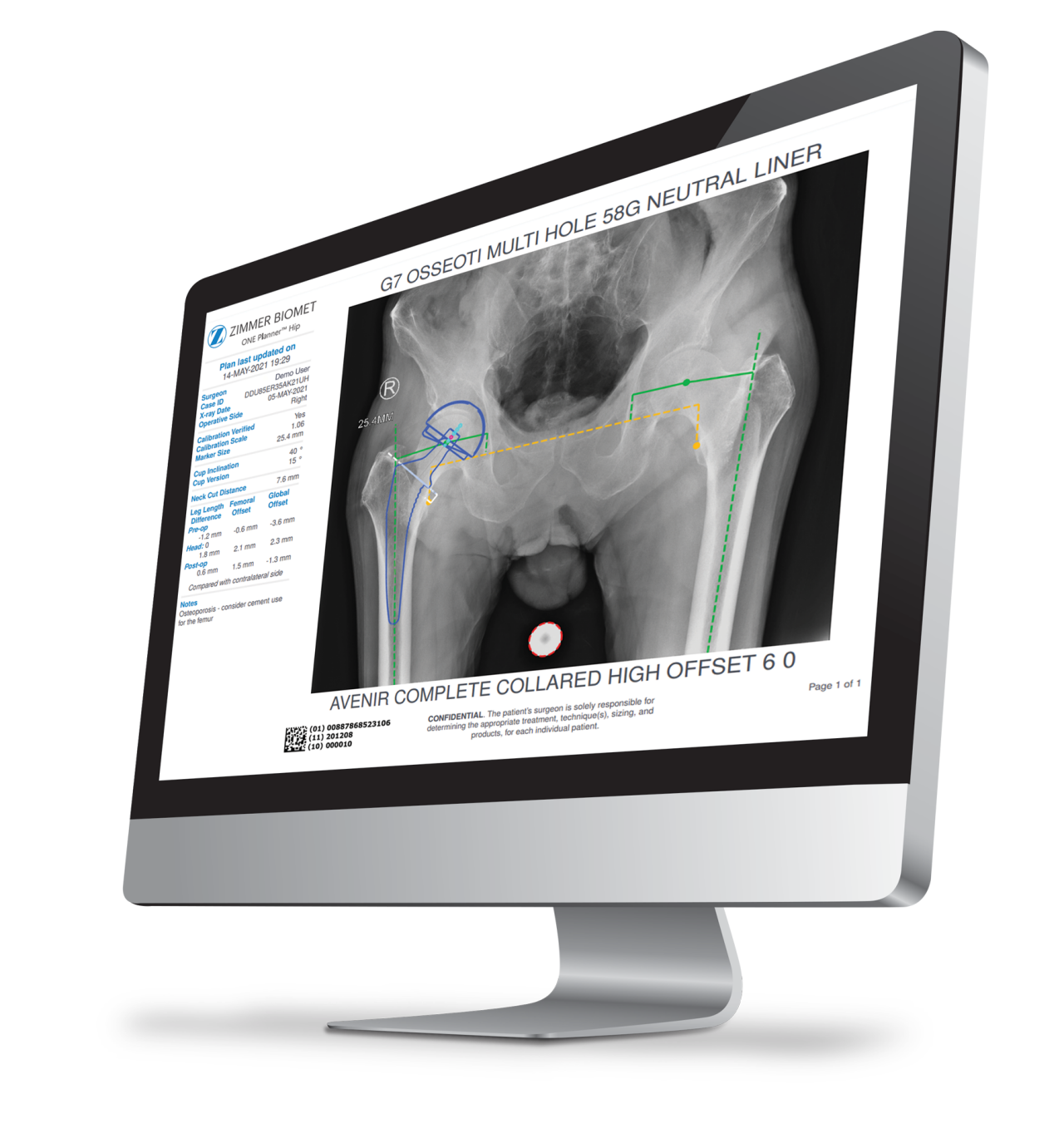
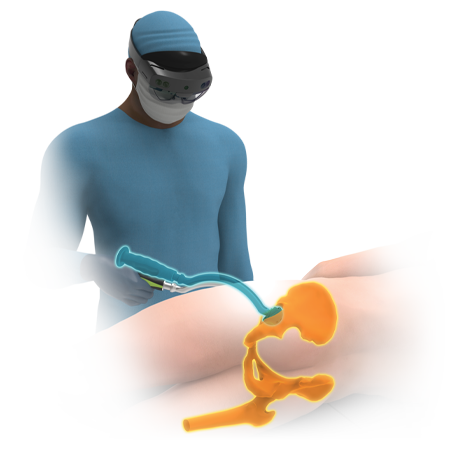
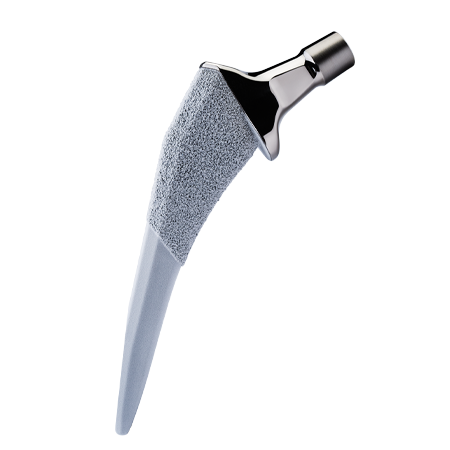
From joint preservation to complex revision arthroplasty, Zimmer Biomet offers a comprehensive portfolio of hip products that combine rich clinical heritage with modern technological advancements. Each of these is designed to address the distinct needs of individual patients, while simplifying surgical workflow.
Hip Technologies
Our expansive technology portfolio unites the latest innovations in biological fixation, advanced bearing materials and infection diagnosis to offer surgeons and hospitals the ability to meet the various needs of today’s patients.
Hip Fracture
Zimmer Biomet addresses the unpredictability of fracture cases by offering femoral and acetabular options with system-based platforms to treat various patient anatomies and bone quality.
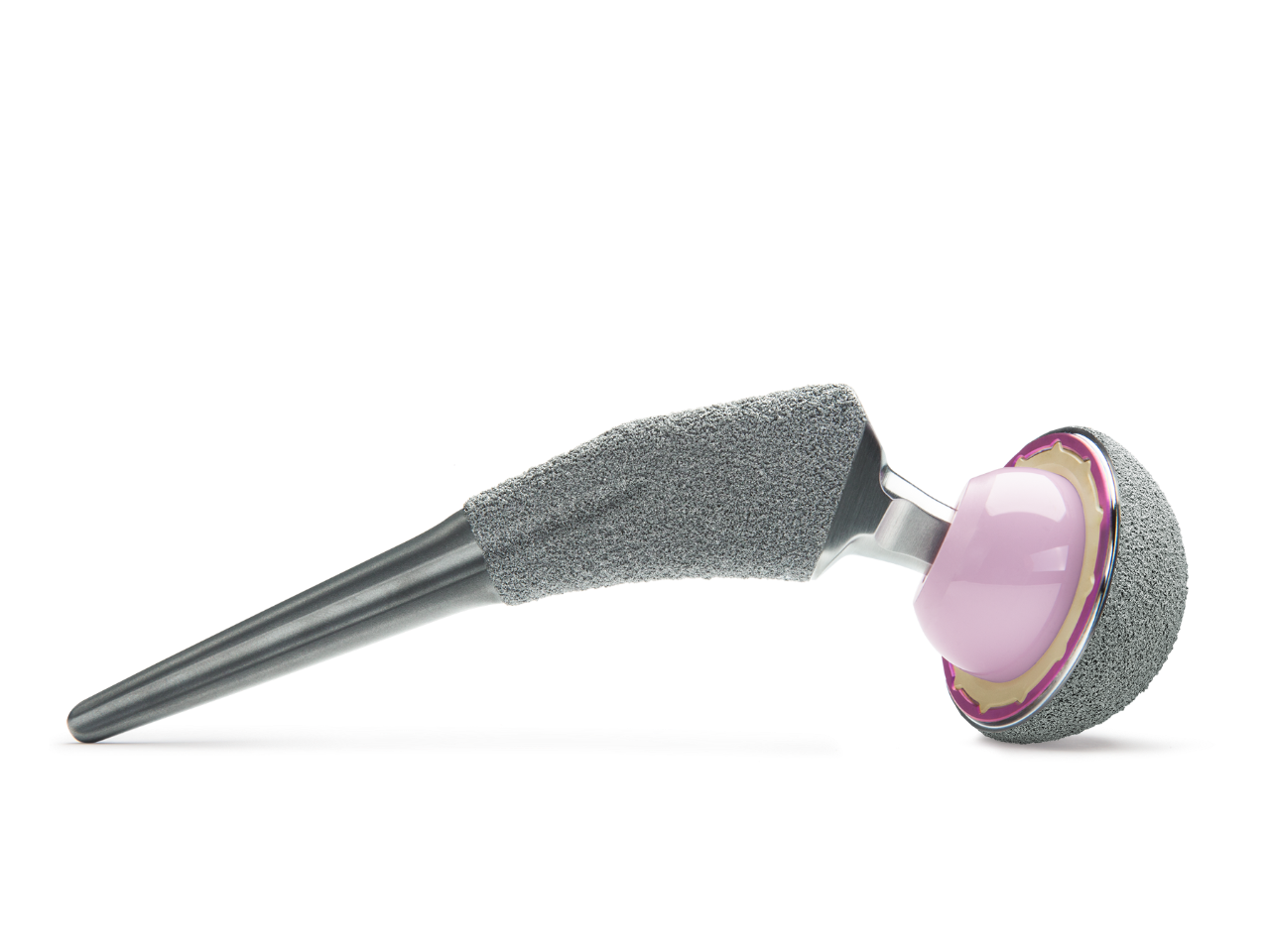
Total Hip Arthroplasty
The Zimmer Biomet primary hip portfolio encompasses femoral and acetabular systems to address the specific needs of each patient and implant designs to accommodate the surgeon’s chosen philosophy.
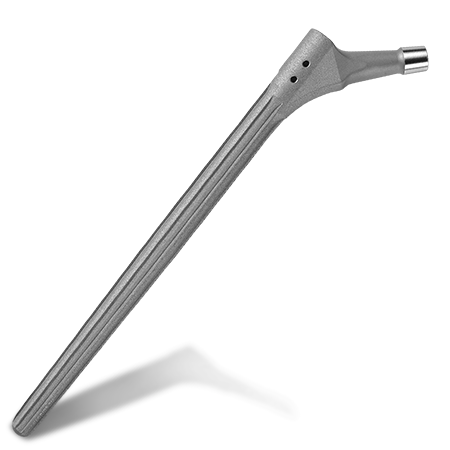
Femoral Reconstruction
Additional Product Information
Acetabular Reconstruction
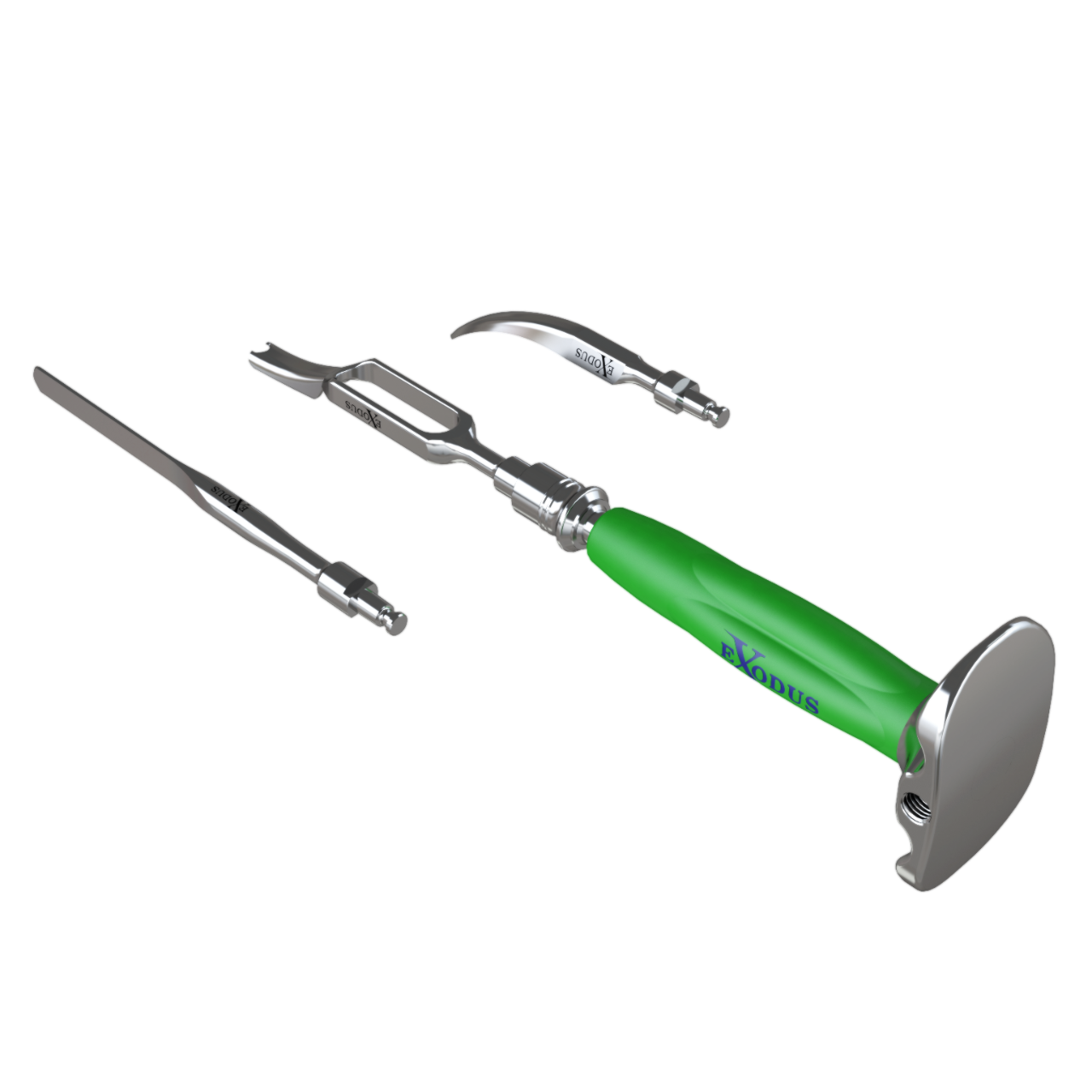
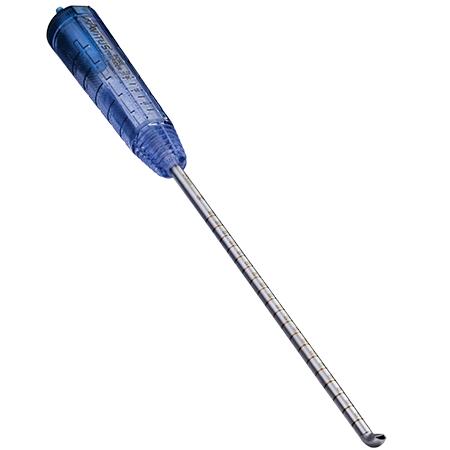
Instrumentation
Revision Hip Arthroplasty
Our comprehensive revision hip arthroplasty portfolio simplifies the complexity of difficult primary and revision THA. This includes femoral, acetabular and auxiliary options that accommodate a wide variety of bone conditions to provide individualized solutions for patients.
Femoral Reconstruction
Acetabular Reconstruction
Patient-Matched Hip Implants
Anatomic individually or extremely difficult reconstruction cases may require the development of a patient-specific hip implant. In such cases, Zimmer Biomet’s Patient-Matched Implant (PMI©) department is available to work directly with surgeons to address the most severe cases of bone loss or deformity. Using CT, X-Ray or MRI data, the PMI team can provide can provide a possible solution.
Robotics
ROSA® Hip is a personalized robotic system that enables DA surgeons to evaluate and execute a surgical plan based on real-time feedback and the patient’s unique anatomy.
It provides surgeons with reassurance and control, while seamlessly integrating into their workflow.
Tailored resources for your patients
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.

All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.