Enhances proximal offloading and bone preservation and provides rotational stability
Taperloc® Complete Hip System
Taperloc Complete Hip System builds on the clinically proven Taperloc hip stem.1-3
Launched in 1982, the Taperloc Hip Stem is the longest clinically referenced hip stem with a wedge shape, titanium substrate, and proximally circumferential titanium porous plasma sprayed design.1-3
Procedures
- Total Hip Arthroplasty
- Femoral Reconstruction
Philosophies
- Tapered Wedge
- Cementless
Application
- Primary
Based on the clinically proven Taperloc Hip Stem
29 Year Survivorship
90%(82% – 95%) at 29 years for any reason, any component [Kaplan-Meier]4
10 Year Survivorship
98.34%Kaplan-Meier for stem only5
29 Year Survivorship
100%For aseptic loosening stem [Kaplan-Meier]4
System Features
The Taperloc Complete Hip System has since become the industry standard in cementless hip arthroplasty.1 Combining unmatched clinical success with Zimmer Biomet’s commitment to product innovation, the Taperloc Complete Hip System has design enhancements that are intended to help surgeons restore leg length, stability, offset, and range of motion accurately and consistently. The Taperloc Complete Hip System comprises of the following femoral prosthesis offerings:
Taperloc Complete Femoral Stem
Features a reduced distal geometry in which a gradual reduction of the stem substrate occurs distal to the porous coating level
Taperloc Complete Microplasty® Femoral Stem
Features a reduced stem length from the standard length stem to better address minimally invasive techniques, providing an alternative to femoral resurfacing while offering a solution in cases where a bone conserving prosthesis is desirable
Taperloc Complete XR 123° Femoral Stem
Helps address femurs with a more varus neck by allowing for additional offset to properly restore hip biomechanics and soft tissue tensioning
Specifications
Benefits
Clinically Proven
Design built on the Taperloc stem (launched in 1983), the longest clinically referenced1-3 primary hip stem with:
Wedge shape
Proximally circumferential titanium porous plasma sprayed design
Titanium substrate
Designed to Treat a Range of Patient Anatomies
Full Length
Reduced Distal
Microplasty (35 mm length reduction)
Consistent Sizing (1 mm increments)
Designed to provide optimal interchangeability, O.R. efficiency, and accurate matching of the patient's femur without the need to remove additional bone
Technology
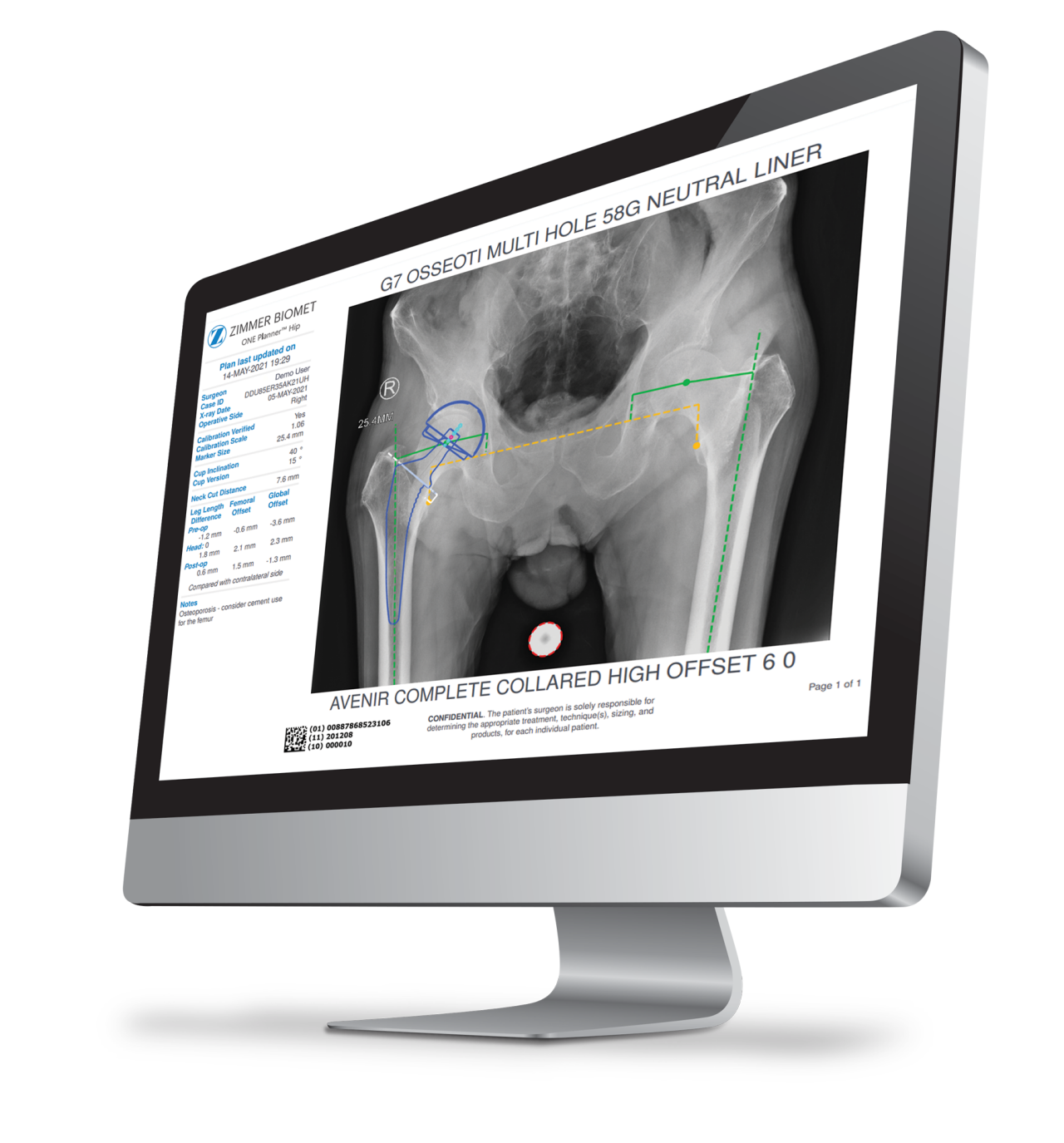
The Taperloc Complete Hip System is compatible with ONE Planner Hip, a web-based preoperative planner for primary total hip arthroplasty. Using the auto-plan mode, surgeons can create a preoperative plan within five minutes.
ONE Planner Hip features an optional spinopelvic mobility assessment tool if both a sitting and standing lateral x-ray are provided with the AP X-ray. This web-based planner requires no onsite installation and adds no extra steps to the workflow.
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.