HAMMR® Automated Hip Surgical Impactor System
Accelerated Precision
HAMMR Brand Anthem Video

ADJUSTABLE.
POWERED.
PRECISE.
The HAMMR Automated Hip Impaction System is designed to address surgeon strain, fatigue, and repetitive motion associated with the traditional mallet during total hip arthroplasty.

ADJUSTABLE
- Three adjustable energy levels to effortlessly address bone quality variation in both the femur and acetabulum.
POWERED
- Ergonomic and balanced impaction device designed to replace the traditional mallet to address fatigue and repetitive motion.
PRECISE
- Consistent, controlled and precise energy1 designed to minimize variability in both bone preparation and device implantation.
Designed for multiple surgical approaches and implant systems.
Adapters are available for anterior and posterior hip replacements and are secured to the device during use to address off-axis impaction that can occur with a mallet.

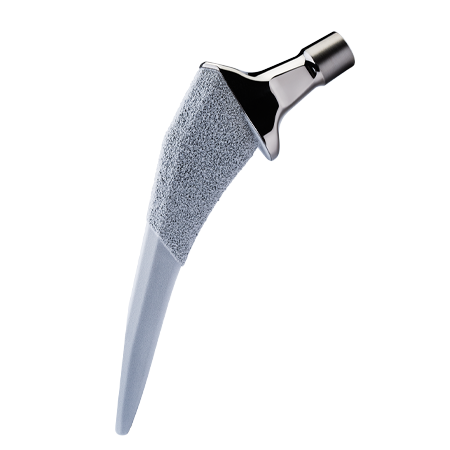
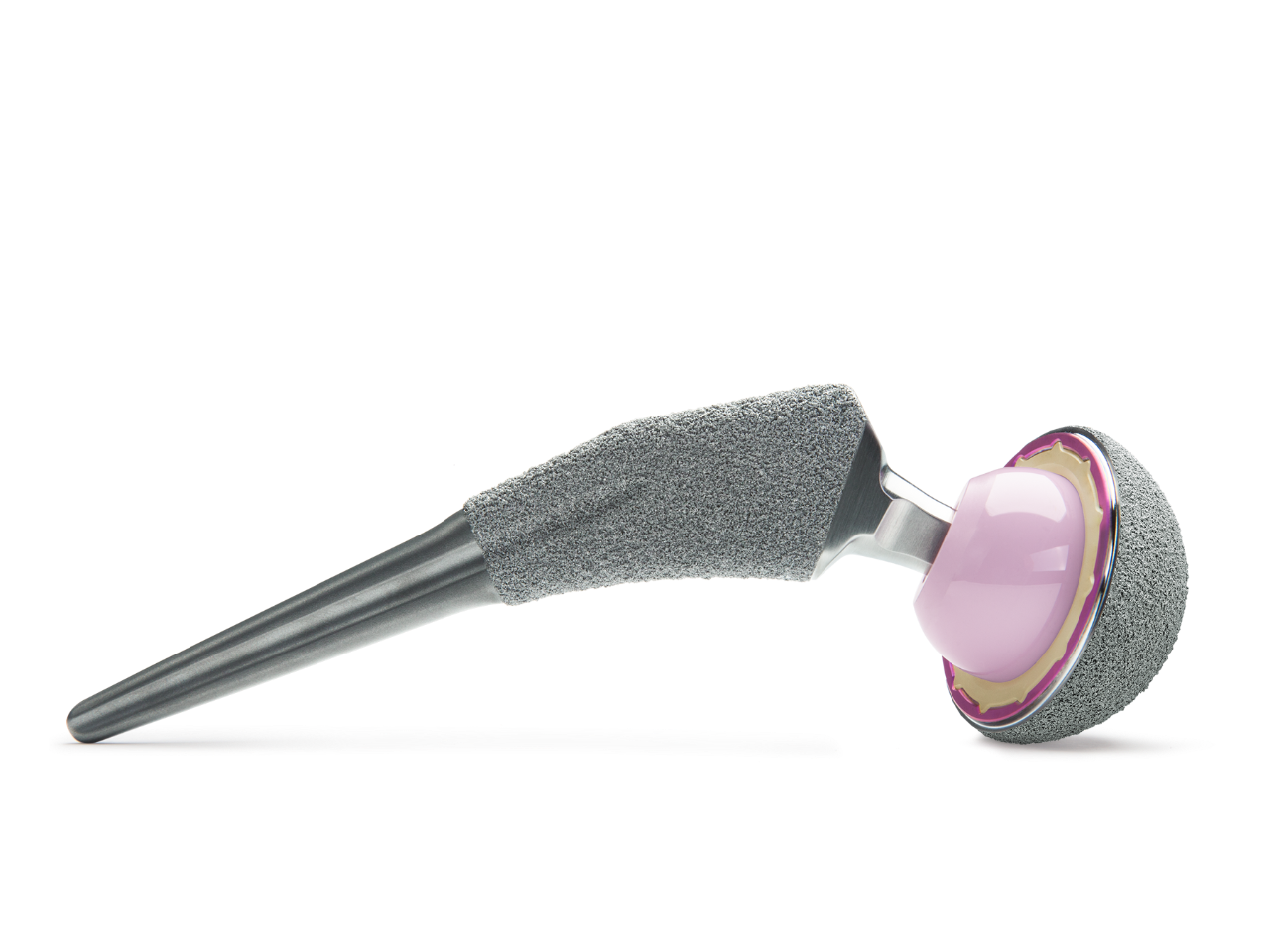
HAMMR pairs seamlessly with Zimmer Biomet’s premier hip implant systems.
Don't miss out.
Sign up to learn more about how you can improve your hip surgical experience with HAMMR.
*The HAMMR automated impaction system is contraindicated for use with the size 7 Echo® Bi-Metric® Full Length High Offset stem.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.