Enables intraoperative adjustment without lengthening the leg
The Echo Femoral Hip System offers a modern metaphyseal loading fit and fill design to address increasing hospital demands for both primary and fracture applications.
Procedures
Philosophies
Application


The Echo® Bi-Metric® System represents the union of two clinically proven Zimmer Biomet implants: the Integral® and Bi-Metric femoral components. Backed by this long standing clinical heritage, the Echo Bi-Metric System enhances existing features of each and offers benefits to better meet patient demands.4-7
Addresses multiple anatomies, to suit various surgical approaches.
Including 2 reduced proximal profile sizing options to accommodate varying anatomies
To maximize fit in smaller femoral canals or Dorr Type C femurs, reducing the need to force or undersize the implant to achieve the desired press fit.


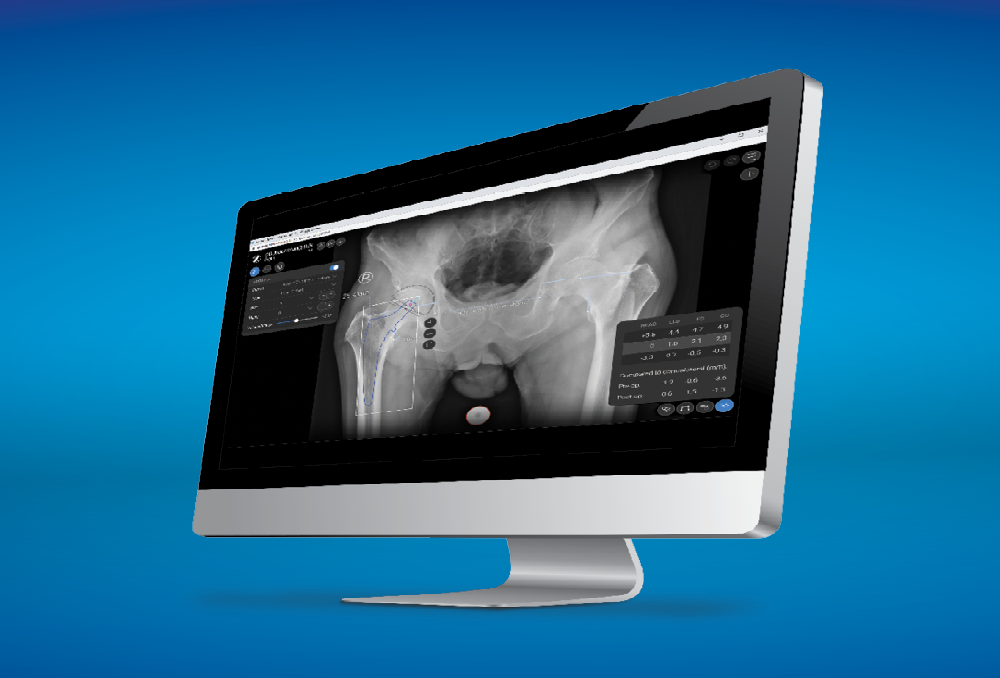
The Echo Hip System is compatible with ONE Planner Hip, a web-based preoperative planner for primary total hip arthroplasty. Using the auto-plan mode, surgeons can create a preoperative plan within five minutes.
ONE Planner Hip features an optional spinopelvic mobility assessment tool if both a sitting and standing lateral x-ray are provided with the AP X-ray. This web-based planner requires no onsite installation and adds no extra steps to the workflow.
Literature
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.