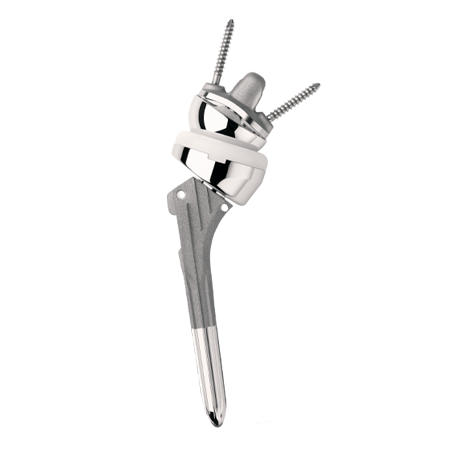
Zimmer Biomet offers a complete array of shoulder replacement systems and associated products. As well as being optimized for the sizing of each patient’s unique anatomy, all of our shoulder systems address key surgeon needs so that joint reconstruction, range of motion, and kinetics may be restored, to help you get patients back to the activities of daily life. From modular anatomical shoulder implants, to industry-leading reverse shoulder arthroplasty prostheses, our shoulder replacement products allow surgeons to devise and execute a patient-specific surgical plan with precision.
Innovative Products
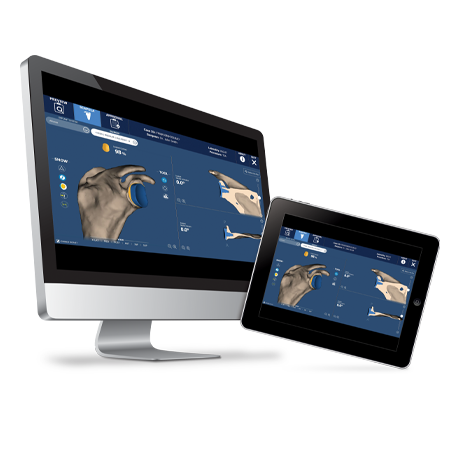
For over four decades, we have been at the forefront, developing Extremities products designed to deal with whatever challenge might face the orthopedic surgeon. Through shoulder systems such as the Bigliani/Flatow® Complete Shoulder, Anatomical Shoulder™ System, Trabecular Metal™ technology, and Comprehensive® Shoulder Systems to technologies such as Signature™ ONE Planner and Patient-specific instrumentation (PSI), innovations in shoulder arthroplasty have always been a focus.
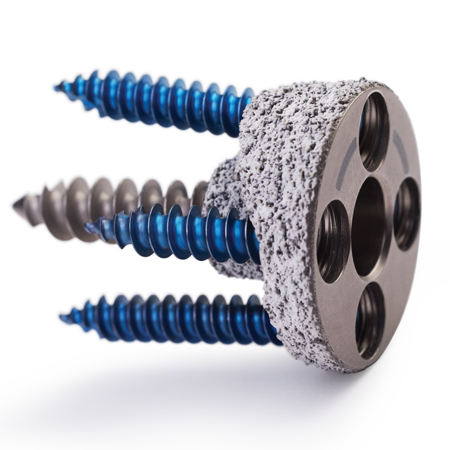
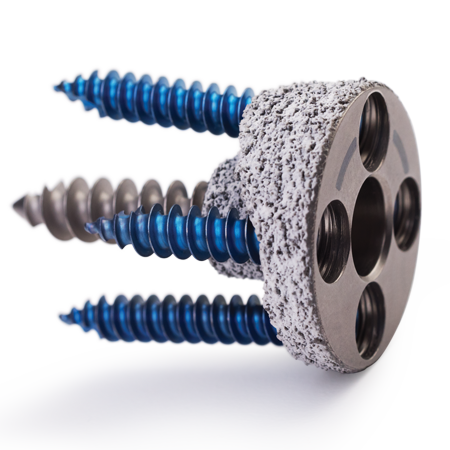
That legacy continues today with products such as the Sidus® Stem-free Shoulder System for true stemless shoulder arthroplasty, the surgeon planned and patient-matched manufactured Vault Reconstruction System (VRS), the Comprehensive Augmented Baseplate for patients with glenoid bone deficiency, and Comprehensive Mini Humeral Trays for optimal fit and function.
Robotics
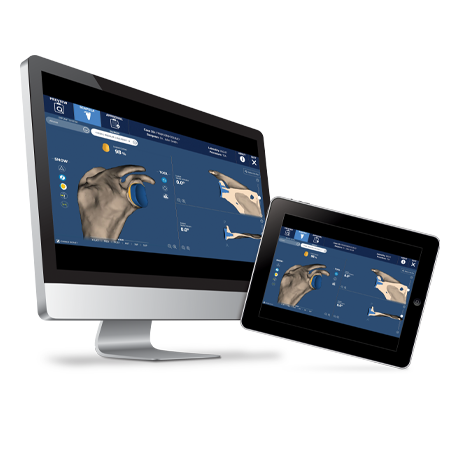
Enable greater precision and accuracy1 with ROSA® Shoulder, the groundbreaking robotic system for anatomic and reverse shoulder arthroplasty.
Anatomic Shoulder
The modularity of Zimmer Biomet’s anatomic shoulder system makes it fully customizable and very versatile. Available in a wide range of sizes, it has been designed for strong fixation. New, easy-to-use instrumentation give it the flexibility to move from a hemi-arthroplasty to a total or reverse arthroplasty, or revise if necessary.
Reverse Shoulder
Zimmer Biomet understands the demands put on reverse shoulder systems. We’ve engineered innovation into each component of our reverse shoulder systems. The task of repairing complex fractures, or restoring as much range of motion as possible so patients with rotator cuff deficiencies can return to daily activities can be challenging.
Fracture Shoulder
Zimmer Biomet provides a wide range of fracture repair options that help to restore joint stability. Our products are used to replicate the proximal humerus regardless of severity of the fracture. Our fracture stems come with unique design variables, such as hashmarks on the implant for consistent height from preparation, trialing to final implantation of the Comprehensive® Fracture Stem, or fracture spikes on the Anatomical Shoulder™ Fracture Stem to provide tuberosity stability with the goal for long-term healing. Both systems can be configured as a reverse shoulder to address rotator cuff deficiency.
Revision Shoulder
To assist with the most challenging arthroplasty surgeries, Zimmer Biomet offers the Comprehensive® Segmental Revision System (SRS) to provide proximal, distal or total humeral replacement options. In the event of severe bone loss requiring total humeral replacement, the system mates with our Nexel® Total Elbow System ulna components. Proximally, the system can be configured for anatomic or hemi shoulder replacement, or in cases of rotator cuff deficiency, it can be configured as a reverse shoulder.
Personalized Solutions
Zimmer Biomet’s mission is to help you help patients move beyond pain and regain function. With this in mind, we’re dedicated to advancing available technologies for shoulder arthroplasty. Our shoulder technology products offer surgeons powerful tools designed to promote surgical efficiency, allow for intraoperative flexibility, and address a variety of patient-specific needs.
Other Products
Zimmer Biomet has been helping surgeons with their shoulder arthroplasty needs for over 40 years. Aside from investing in new technologies to potentially improve patient outcomes, we are committed to providing treatment options with a long track record of clinical success. Our Trabecular Metal™ material is designed for long-term fixation. The Bigliani/Flatow® Shoulder System provides a simple, reproducible treatment for osteoarthritis. With such a broad shoulder arthroplasty portfolio, Zimmer Biomet can meet your every need.
Tailored resources for your patients
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.

All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.