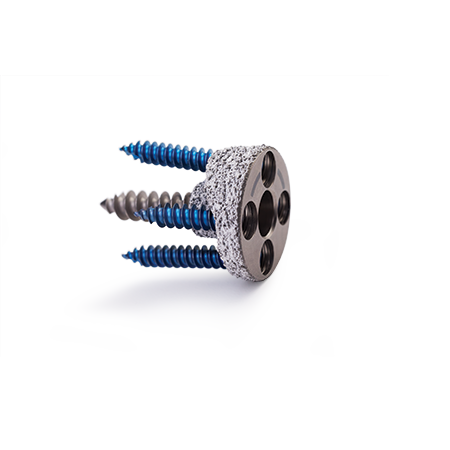
Comprehensive® Reverse Shoulder System Augmented Baseplate
Augmented Baseplate
Up to 50% of patients presenting with osteoarthritis and associated rotator cuff arthroplasty have bone erosion that must undergo reconstruction in order to achieve successful shoulder arthroplasty.1 The use of an augmented baseplate provides a simple, reproducible method to restore the glenoid for reconstruction in Reverse Shoulder Arthroplasty. For patients with a lesser degree of bone loss or correction, an augmented baseplate can add benefits of less bone removal, preservation of cortical bone, and lateralization.*


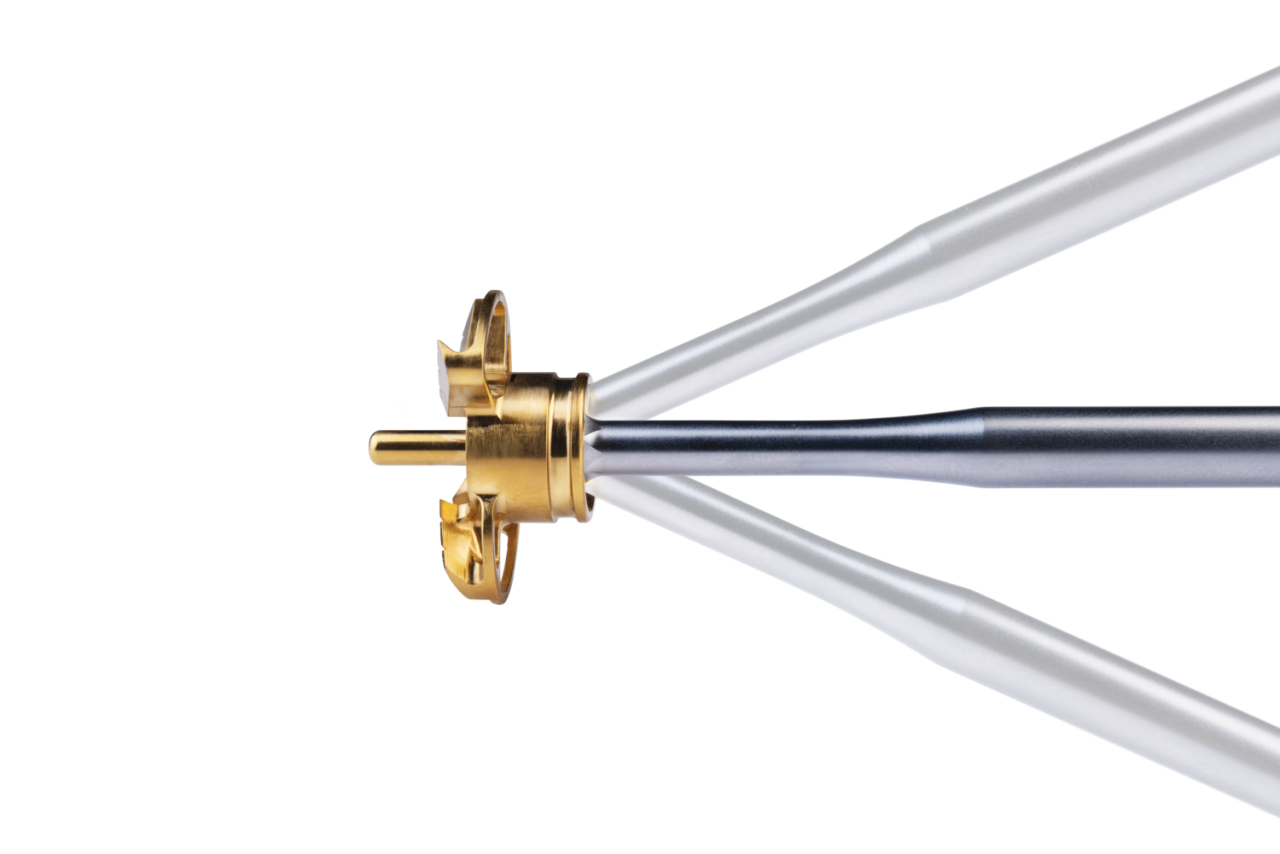
Bone Preparation
Comprehensive® Augmented Baseplate instrumentation now features Off-Axis reaming technology, which allows for glenoid preparation to be executed at a broad range of angularity. A simplified, reproducible bone preparation solution designed to be exposure-friendly while facilitating control and visibility during the ream process.
Off-Axis reaming technology is intended to support reliable and consistent augment placement to facilitate stability for glenoid component fixation, which is critical to the long-term success of reverse shoulder arthroplasty.2,3
Key benefits and features of Off-Axis reaming:
- Ball hex technology for reaming functionality at 30 degrees in any direction
- Low-profile reamer guides designed to help fix rotational position
- Modularity for intraoperative, in-situ assembly
Product Features
Technology
- Modular 6.5 mm central compression screw for enhanced stability
- Central boss for sharing the sheer load being transferred to the glenoid
- Specialized etch on baseplate face to facilitate accurate augment placement
- Circular Baseplate allows augment to be positioned to any orientation around glenoid face
- HA/TCP over PPS® coating for fixation.
Videos
Comprehensive Reverse Shoulder System Augmented Baseplate Surgical Technique Animation
Watch animation
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.