Tapestry® Biointegrative Implant
Elevating Tendon Augmentation
A Biointegrative Implant
The Tapestry Biointegrative Implant has a highly aligned and porous structure with consistent microarchitecture, similar to native tendon1 and gradually resorbs, leaving new tendon-like tissue in place of the implant2.
Product Features
Mimics Native
Tissue
- Tapestry’s highly aligned and highly porous structure is similar to native tendon.1
- Specifically designed to support tendon healing, Tapestry demonstrated high cellular attachment in vitro as early as 8 days,3 enabling tendon-like tissue ingrowth.
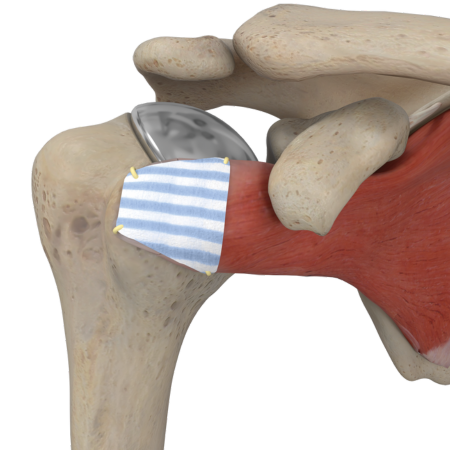
Biointegrative
Implant
- Over time, Tapestry resorbs, leaving new, dense, collagenous connective tissue around and in place of the implant.2
- Tapestry is designed for bioresorbable healing, demonstrating high cellular attachment in vitro,3 facilitating tendon-like tissue ingrowth.
- In a pre-clinical study, Tapestry demonstrated complete integration and formation of dense collagenous fibrous connective tissue into and around the implant as early as 8 weeks.2

Porous
- Tapestry supports optimal fluid absorption, cell attachment and proliferation, and vascularization3,4, enabling the formation of new tendon-like tissue.
- With tailored fiber diameter and specific porosity, Tapestry’s microarchitecture includes 38% of Tapestry’s pore volume within the range of 50-300μm.5
- Tapestry can absorb 37x its weight in blood in 30 seconds compared to Regeneten, which absorbs 3x its weight in 30 seconds.6
Videos
Tapestry® Biointegrative Implant Brand Anthem Video
Tapestry® Biointegrative Implant Testimonial - Dr. Jeffery Declaire
Additional Information
Related Products
Want to learn more? Contact us here.
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
Tapestry Biointegrative Implant is not indicated to replace damaged tendons or to reinforce the strength of any tendon repair.
Regeneten is a trademark of Smith & Nephew.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.




