The Power to Personalize™
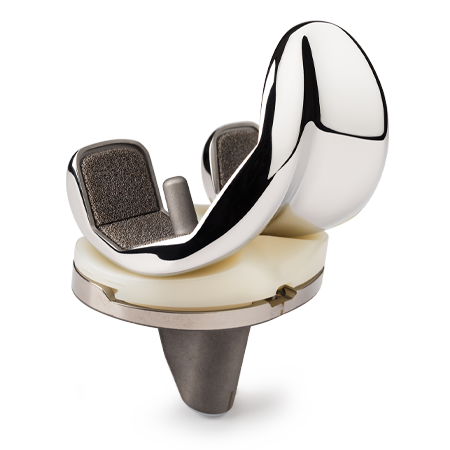
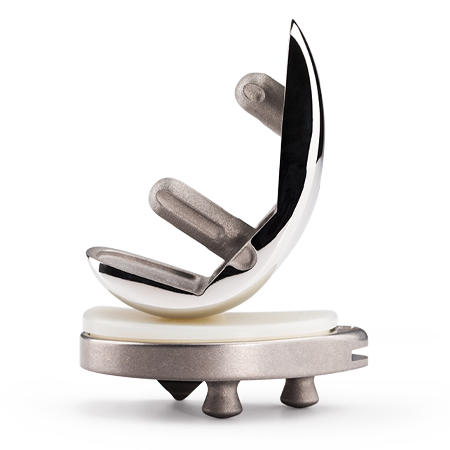
Persona® Revision Knee
Personalized Implants, Precise Instrumentation, Proven Technology.
The Persona Revision Knee System expands the Persona Knee System to provide a portfolio of personalized knee care in order to replicate a patient’s anatomy and offer an individualized fit in partial, primary and revision knee arthroplasties.
Procedures
- Knee Reconstruction
- Revision Knee Reconstruction
Philosophies
- Anatomic Reconstruction
- Zonal Fixation
- Soft Tissue Balance
Application
- Revision Knee
The Persona Revision Knee System provides proven1-3 solutions to help improve patient outcomes
Trabecular Metal Technology
20 yearsPersona Revision boasts one of the most clinically proven1-3 biological fixation portfolios on the market with over 20 years of successful clinical use.
Clinical Heritage
55 yearsPersona Revision Knee System is an evolutionary refinement of its predecessors: Persona the Personalized Knee, NexGen® Legacy® Constrained Condylar Knee (LCCK), Natural-Knee® II, and Vanguard® SSK/360 Revision Knee System. The design and material properties of these systems have a combined clinical history of over 55 years on the market.4-7
Bonus Coverage with Proper Rotation
92%In vitro, the Persona Anatomic Tibia has demonstrated 92% bone coverage with proper rotation8


System Features
The Persona Revision Knee System’s personalized implants, precise instrumentation and proven technologies give surgeons the ability to personalize their surgical experience to best meet the needs of each patient.
Personalized Implants
Achieve an individualized fit with an array of anatomic components including tibial and femoral cones and numerous stem choices to address fixation needs.
Precise Instrumentation
Supports a personalized approach that allows surgeons to individualize the surgical experience based on the patient’s bone and joint condition.9-12
Proven Technology
Boasts one of the most clinically proven1-3 biological fixation portfolios on the market with premium bearing technology designed to protect against oxidation and maintain wear resistance.9-12
Specifications
Benefits
Zonal Fixation
- Persona Revision Trabecular Metal Cones provide a solution to effectively fill bone defects to closely replicate patient’s normal anatomy. The proven1-3 Trabecular Metal material facilitates biological fixation and distributes loads closer to the joint line.
Soft Tissue Balance
- In an effort to address joint instability, Persona Revision’s multiple femoral components in varying sizes and a vast continuum of bearing constraints provide surgeons the tools to help achieve a stable knee.
Streamlined Delivery System
- Efficient kitting strategy with modular brackets allows for adaptable tray configurations to help eliminate nonessential instrumentation.
Videos
Revision Knee Arthroplasty: Anatomic Restoration with the Persona® Revision Knee System
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.