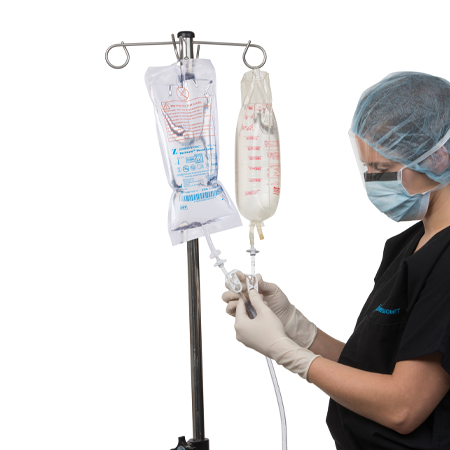
Bactisure® Wound Lavage
Advancing Removal of Debris
Bactisure® Wound Lavage is used with the Zimmer Pulsavac® Plus or Pulsavac Plus AC Wound Lavage Systems, and is indicated for use in cleansing and removal of debris, including micro organisms, from wounds.
Removes
Bactisure Wound Lavage facilitates easy removal of microorganisms via pulsed lavage.
Exposed bacteria are subject to lavage removal or inactivation via traditional antibiotics and the body’s normal defense mechanisms.1
System Features
Key Application Steps
- Apply prior to wound closure using Zimmer Biomet Pulsavac Plus pulsed lavage system.
- Immediately after irrigating the wound bed, the wound bed MUST BE rinsed away with an equal amount of normal saline.


Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.