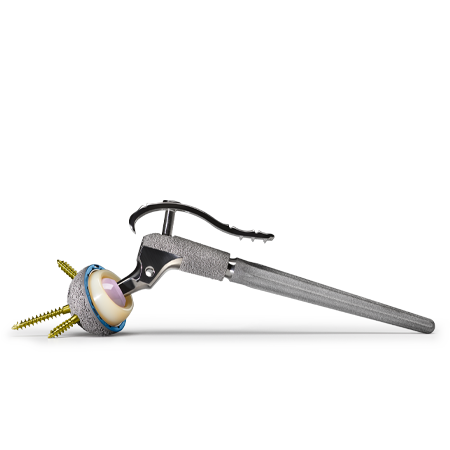
Setting the standard for acetabular reconstruction
Trabecular Metal® Acetabular Revision System
Breaking the Revision Cycle
The Zimmer Biomet Trabecular Metal Acetabular Revision System (TMARS) combines clinically proven Trabecular Metal Technology1–3 with the ability to tailor individualized solutions for each patient. From simple to complex revision arthroplasty, this combination sets the standards for acetabular reconstruction.
Procedures
- Acetabular
Reconstruction - Revision Hip
Philosophies
- Cementless
Application
- Revision
An algorithmic approach to complex revisions
10 years
99%Survivorship at 10 years when a TM Revision Shell is used in revision THA.4
System Features
The TMARS system consists of the following acetabular and auxiliary implant designs:
Trabecular Metal Revision Shells and Liners
Designed for use in revision and primary cases. A one-piece construct, created by cementing the liner into the shell, eliminates concerns about backside wear. The low modulus of elasticity of the Trabecular Metal material can produce more normal physiological loading and reduce stress shielding.5
Trabecular Metal Cup Cage
Used in conjunction with the Trabecular Metal Revision Shell to provide adequate stability by spanning acetabular defects and pelvic discontinuities to provide initial mechanical stability of the Cup-Cage construct until biological fixation occurs. Cementing the Longevity Highly Crosslinked Polyethylene Liners, Cages, and Trabecular Metal Revision Shells together creates a single construct, without concerns of micromotion.6
Trabecular Metal Buttress and Shims
- Work with the revision shell to address extensive superior segmental defects (Paprosky Type IIIA).
- Serves as an alternative to allograft, without potential for bone resorption or disease transmission.
- Creates a monolithic construct without concerns for micromotion by using cemented interfaces.6
- Conserves host bone while implant size, position, and orientation are determined by the defect.
Trabecular Metal Augments and Restrictors
The Trabecular Metal Acetabular Augments and Restrictors fill bone deficiencies as an alternative to preparing and using structural grafts.7

Specifications
Benefits
Proven
- Proven technology and fixation
Addresses Range of Deformities
- Designed to fit Paprosky Type I to IV acetabular deformities, enabling an algorithmic approach to challenging revisions.9
Lower Infection Rates
- Trabecular Metal (TM) acetabular components used in revision THA are associated with decreased rates of re-revision for infection compared to non-TM components as reported by Tokarski et al.10
Education
Literature
Videos
Trabecular Metal™ Acetabular Revision System (TMARS) Surgical Demonstration
Watch Video
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.