Compatible with most Zimmer Biomet heads and adapters**
Avenir Complete® Hip System
Proven Heritage.1
Operative Flexibility.
Surgical Excellence.
The Avenir Complete Hip System is an evolution based on the clinically proven heritage of the Avenir Hip System.2-5
Procedures
- Total Hip Arthroplasty
- Femoral Reconstruction
Philosophies
- Bone Compaction
- Cementless
Application
- Primary
System Features
Built on the legacy of the Avenir Hip System1-5
Specifications
Benefits
Proven Heritage
- The Avenir Complete Hip System is an evolution based on the clinically proven heritage of the uncemented Avenir Hip System2-5. By combining legacy features of the Avenir Hip System with new features, Avenir Complete was designed to deliver greater operative flexibility along with surgical excellence.
Operative Flexibility
- As your trusted partner in Anterior Hip surgery, we know that stem selection is of prime importance when performing the approach. This doesn’t just mean a shortened stem for ease of insertion, but a stem that provides greater operative flexibility to best match patient anatomies and help ensure proper fit within the femur.6 The Avenir Complete Hip System was designed to deliver greater operative flexibility through an expanded feature-set. This expanded feature-set includes optimized stem lengths, a reduced distal geometry, a simple 6mm offset system with three offset options (Standard, High Offset & Coxa Vara) and streamlined instrumentation designed to achieve accurate and precise stem seating.
Surgical Excellence
- Fostering Operating Room efficiency and reproducible surgical technique, together with facilitating accurate and precise stem seating. Includes a streamlined instrumentation set, various broach handles are available to accommodate modern surgical approaches including the muscle sparing anterior approach. The line-to-line relationship between the final broach and implant is designed to achieve accurate and precise implant seating with regard to the position of the final broach and resection line helping to manage leg length.

Technology
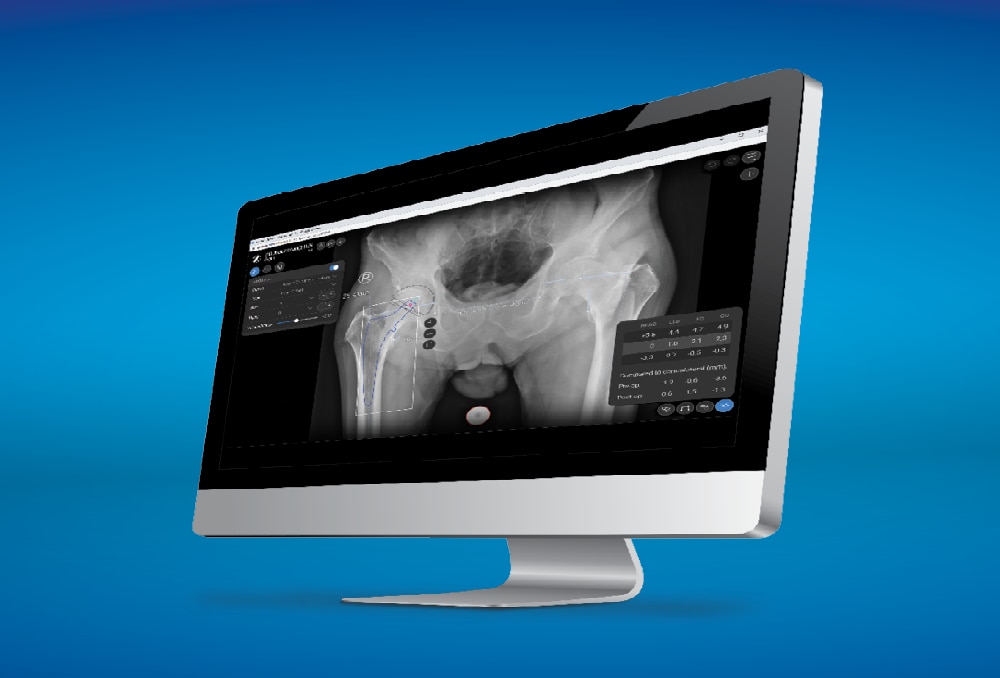
The Avenir Complete Hip System is compatible with ONE Planner Hip, a web-based preoperative planner for primary total hip arthroplasty. Using the auto-plan mode, surgeons can create a preoperative plan within five minutes.
ONE Planner Hip features an optional spinopelvic mobility assessment tool if both a sitting and standing lateral x-ray are provided with the AP X-ray. This web-based planner requires no onsite installation and adds no extra steps to the workflow.
Education
Literature
Videos
Avenir Complete Product Overview
Watch animation
Avenir Complete Hip Surgical Technique
Watch animation
A Partner for the Anterior Approach feat. Dr Yerasimides
Watch animation
My Early Experience feat. Dr Yerasimides
Watch animation
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.






