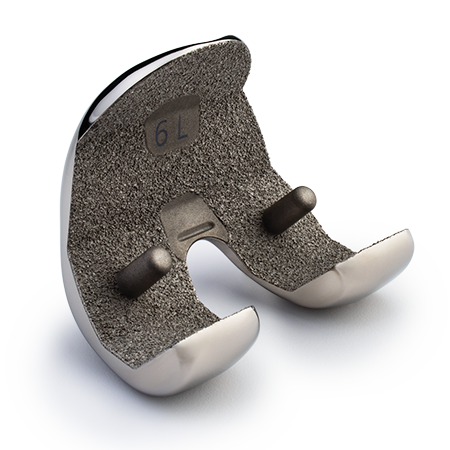
Persona® Revision
SoluTion™ Femur
It's not just a femur.
It's a SoluTion.
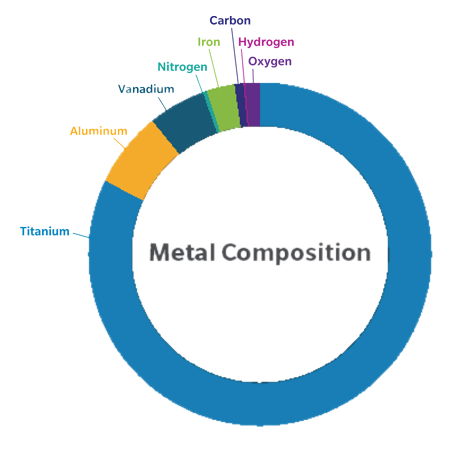
The Persona® Revision SoluTion™ Femur may help address certain metal sensitivities with a durable1,2, scratch resistant femoral component virtually free of Nickel, Cobalt, and Chromium, part of the comprehensive Persona Knee System.
Metal Sensitivity in Patients
Revision TKA (rTKA) is one of the most complex orthopedic procedures5 and unexplained pain can represent as much as 10% of all cases.3 Additionally, the prevalence of metal hypersensitivity in the general population is estimated to be around 10-15% while prevalence in patients with metallic implants may be as high as 25%.4 With the Persona Revision Total Knee Arthroplasty (rTKA) SoluTion Femur, having the option of an alternative metal implant may avoid certain potential adverse reactions.5
Benefits
Features
Anatomic tibia and cones
- The revision SoluTion femur includes all the value of the Persona Knee System paired with an anatomic tibia to provide proper rotation and optimal bone coverage10
Premium Bearing Technology with full continuum of constraint
- Compatible with Posterior Stabilized (PS) Bearing, Constrained Posterior Stabilized (CPS) Bearing, Constrained Condylar Knee (CCK) Bearing
Compatible with Vivacit-E®
- Designed with antioxidant protection to help meet long-term performance needs, Vivacit-E provides: exceptional oxidative stability11, ultra-low wear12 and improved strength.13,14

Videos
Hardening Process Animation
Persona® Revision SoluTion™ Femur - Dr. Max Courtney
SUBMIT YOUR INFORMATION AND GET CONTACTED BY A ZIMMER BIOMET REP
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.