It’s not just a femur. It’s a SoluTion.
Persona® SoluTion™ PPS® Femur for Cementless Total Knee Arthroplasty (TKA)
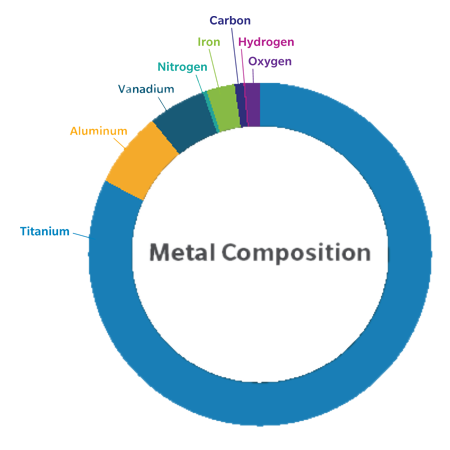
Metal and Bone Cement Sensitivity in TKA Patients
The Persona SoluTion PPS Femur is an alternative metal knee implant for patients who are sensitive to certain metals or bone cement, designed with clinically proven cementless1 and surface-hardening technology2
Metal sensitivity is an immunological reaction of the body against metallic particles (ions) that are released because of implant wear from many common knee implants, such as Cobalt Chrome. Bone cement sensitivity has been shown in some total knee arthroplasty (TKA) patients. One study showed almost 1 in 4 patients who received a cemented implant had contact allergies to common materials found in bone cement, such as Gentamicin and Benzoyl Peroxide.3
Benefits
Features
Anatomic Shape
- The SoluTion femur includes all the value of the clinically proven Persona Knee System with an antomic tibia to provide proper rotation and optimal bone coverage6-8
PPS Press Fit Femur
- PPS porous technology, based on the clinically successful legacy Vanguard® PPS porous material technology, allows for initial scratch-fit stability and biologic internal fixation of the femur9-14
Compatible with Vivacit-E®
- Designed with antioxidant protection to help meet long-term performance needs, Vivacit-E provides: exceptional oxidative stability15, ultra-low wear16 and improved strength.17,18
Extensive Sizing Options
- Offers 2 mm increment sizing in standard and narrow and the same full range of sizes as Persona CoCr Femur.
Videos
Hardening Process Animation
Persona® SoluTion™ PPS® Femur Roundtable
SUBMIT YOUR INFORMATION AND GET CONTACTED BY A ZIMMER BIOMET REP
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.