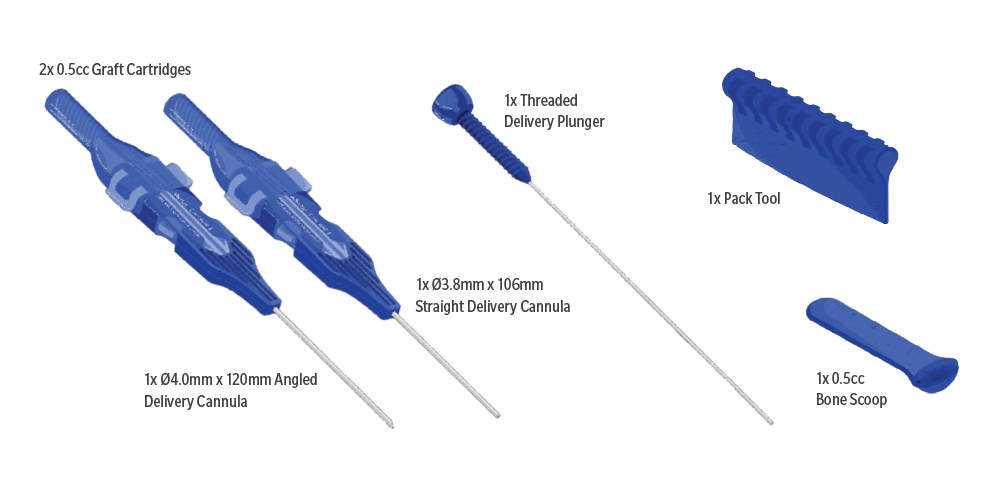
Item | Description | Part No. |
1 | Avitus ArchiMIS Precision Autograft Delivery | AD-1ch-4.0-120 |
Avitus® ArchiMIS™
Precise volume Minimally invasive Controlled delivery
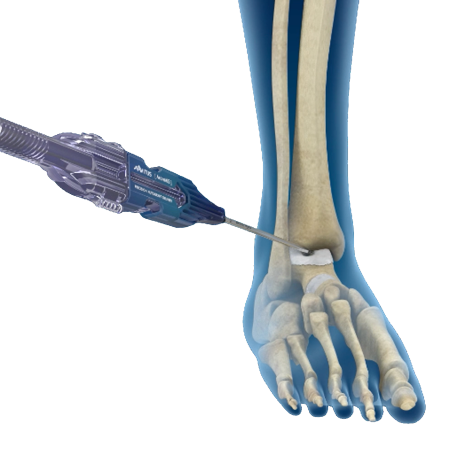
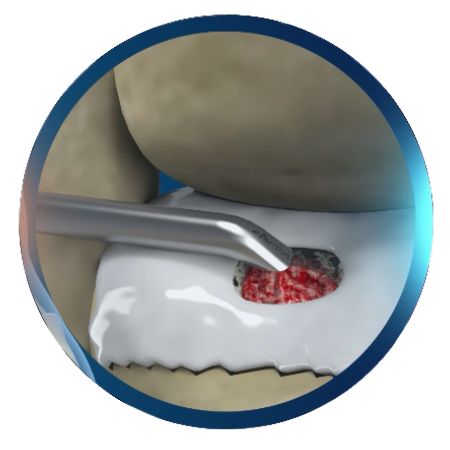
ArchiMIS™ is sterile-packed, single-use delivery kit enabling percutaneous bone graft delivery. Surgeons are able to precisely control small volume bone graft deployment.
Indications for Use:
- To be used for the delivery of autograft or hydrated allograft bone graft material to an orthopaedic surgical site.
Examples:
- OCD bone grafting
- Arthroscopic autograft delivery
- MIS/Percutaneous autograft delivery
- Bone marrow lesions
- MIS/Percutaneous fusions
- Bone lesions and cysts
- Osteochondral lesions
- MIS fusions
System Features
Comprehensive Autologous Bone Graft Delivery
- Straight and curved delivery cannulas available in kit for percutaneous autograft delivery
- Multiple graft cartridges to allow for streamlined delivery process
- Threaded delivery plunger for precise and controlled delivery of bone graft

Specifications
Benefits
Videos
Avitus® ArchiMIS™ Animation
Avitus® ArchiMIS™ Technique Video
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.