- CPT® 2024 Professional Edition. American Medical Association. p. 875
- Calendar Year 2024 Medicare Physician Fee Schedule, Final Rule. Federal Register, November 2, 2023.
- Calendar Year 2022 Medicare Physician Fee Schedule, Final Rule. Federal Register, November 19, 2021.
- Calendar Year 2021 Medicare Physician Fee Schedule, Final Rule. Federal Register, December 28, 2020
Remote Therapeutic Monitoring (RTM) Coding and Payment
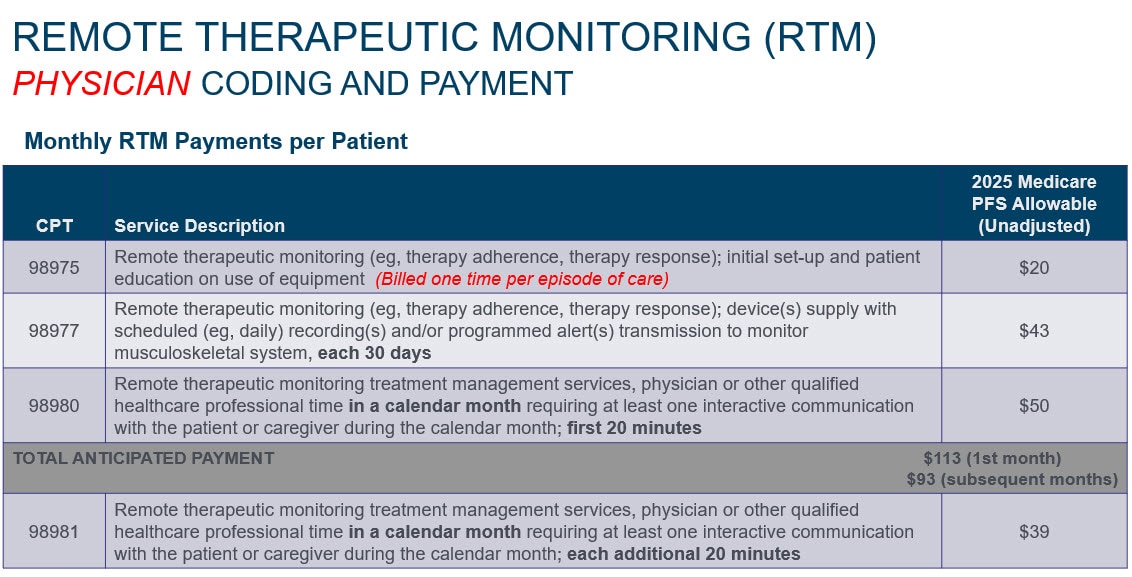
Source: Calendar Year 2025 Medicare Physician Fee Schedule, Final Rule. Federal Register, December 9, 2024
Medicare Coverage Requirements for Reporting Remote Therapeutic Monitoring (RTM):
- RTM services (e.g., musculoskeletal system status, therapy adherence, therapy response, cognitive behavioral therapy, therapy adherence, therapy response) represent the review and monitoring of data related to signs, symptoms, and functions of a therapeutic response. These data may represent objective device-generated integrated data or subjective inputs reported by a patient. These data are reflective of therapeutic responses that provide a functionally integrative representation of patient status.1
- Physicians and eligible qualified health care professionals are permitted to bill RTM as general medicine services. A physician or other qualified health care professional is defined in the CPT Codebook as “an individual who is qualified by education, training, licensure/ regulation (when applicable) and facility privileging (when applicable) who performs a professional service within his/her scope of practice and independently reports that professional service.” Accordingly, RTM codes could be available for physical therapists (PT), occupational therapists (OT), speech-language pathologists, physician assistants, nurse practitioners, and clinical social workers.3
- Remote monitoring codes are designated as care management services and thus CMS’ rules for general supervision apply to these services. ²
- Billing for remote monitoring codes requires data collection for at least 16 days in a 30-day period and applies to the following RTM code: 98977. The 16-day data collection requirement does not apply to CPT codes 98980 and 98981 because these CPT codes are treatment management codes that account for time spent in a calendar month and do not require 16 days of data collection in a 30-day period. ²
- To report 98975 and 98977 the device used must be a medical device as defined by the FDA.1
- Only one practitioner can bill for RPM or RTM (not both) during a 30-day period, and only when at least 16 days of data have been collected on at least one medical device. Even when multiple medical devices are provided to a patient, the remote monitoring services associated with all the medical devices can be billed by only one practitioner, only once per patient, per 30-day period, and only when at least 16 days of data have been collected; and that the services must be reasonable and necessary. ²
- For an individual beneficiary who is currently receiving services during a global period, a practitioner may furnish RTM services (but not both RPM or RTM services) to the individual beneficiary, and the practitioner will receive separate payment, so long as the remote monitoring services are unrelated to the diagnosis for which the global procedure is performed, and as long as the purpose of the remote monitoring addresses an episode of care that is separate and distinct from the episode of care for the global procedure - meaning that the remote monitoring services address an underlying condition that is not linked to the global procedure or service.²
- RTM services being furnished during the global period only applies to billing practitioners who are receiving the global service payment. Practitioners, such as physical and occupational therapists, who are not receiving a global service payment because they did not furnish the global procedure, would be permitted to furnish RPM or RTM services during a global period. ²
- CMS states that self-reported/entered data may be part of the non-physiologic data for purposes of RTM codes. RTM data can be self reported by the patient, as well as digitally uploaded via the device. While RTM codes still require the device used to meet the FDA’s definition of a medical device, self-reported RTM data via a smartphone app or online platform classified as Software as a Medical Device (SaMD) may qualify for reimbursement.3
- Practitioners must obtain consent either in advance or at the time RTM services are furnished and document that consent in the patient’s record.4
- For new patients or patients not seen within the year by billing practitioner, RTM services must be initiated during an in-person visit.4
- RTM services may be provided to patients with either acute or chronic conditions.4
Additional Information
Need additional information? Contact Zimmer Biomet’s Reimbursement Hotline by calling 866-946-0444 or via email at Reimbursement@zimmerbiomet.com.
This website and the material contained herein are intended for informational purposes only. Nothing herein is advice, legal advice or a recommendation of any kind, and it should not be considered as such. The content of this website was obtained from the third-party sources cited within and is subject to change without notice, resulting from changes in reimbursement laws, regulations, rules and policies. All content is informational only, general in nature, and does not cover all situations or all payers' rules or policies. The content is not intended to apply to any particular situation. In all cases, the service and the product must be reasonable and necessary for the care of the patient to support reimbursement. Providers should report the procedure and related codes that most accurately describe the patients' medical condition, procedures performed and the products used. The information on this website represents no promise or guarantee by Zimmer Biomet regarding coverage or payment for products or procedures by Medicare or other payers. This website may not be used as an official source of information. Providers should check Medicare bulletins, manuals, program memoranda, Medicare guidelines and other payer information to ensure compliance with Medicare and other payer requirements. Inquiries may be directed to the Part A/B Medicare Administrative Contractor, or to appropriate payers. Zimmer Biomet specifically disclaims liability or responsibility for the results or consequences of any actions taken in reliance on information in this website.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the package insert and information on this website. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please call 1-800-348-2759, press 4 for 411 Technical Support.