Identity® Revision Stem
Instrumented depth setting and reproducible stem placement for revision and fracture cases.

Designed for Revision and Fracture Cases
Zimmer Biomet is expanding its revision arthroplasty offerings with Identity Revision. A line extension to the Identity Shoulder System, Identity Revision is a long stem designed for both revision arthroplasty and fracture applications where depth setting and reproducible stem placement are focal points. Revision does not deviate from existing Identity workflows and matains modularity and compatibility with Identity components.
Available Diameters and Stem Lengths
- The Identity Revision Stem is 136 mm in length, measured from the distal tip to the intersection of the centerline and the proximal face. For full details about stem specifications, speak to your local Zimmer Biomet representative
- Consistent with the Identity Shoulder System, Revision is available in 15 diametric size options (4 - 18 mm) in 1 mm increments to fit a varying range of patient anatomy
Features and Benefits
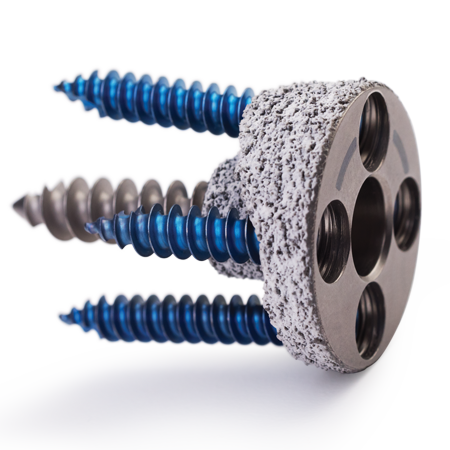
Fixation
- Proven PPS® coating provides for initial scratch fit stability and biological fixation1-3
- PPS is applied circumferentially to approximately 39 mm of the proximal stem
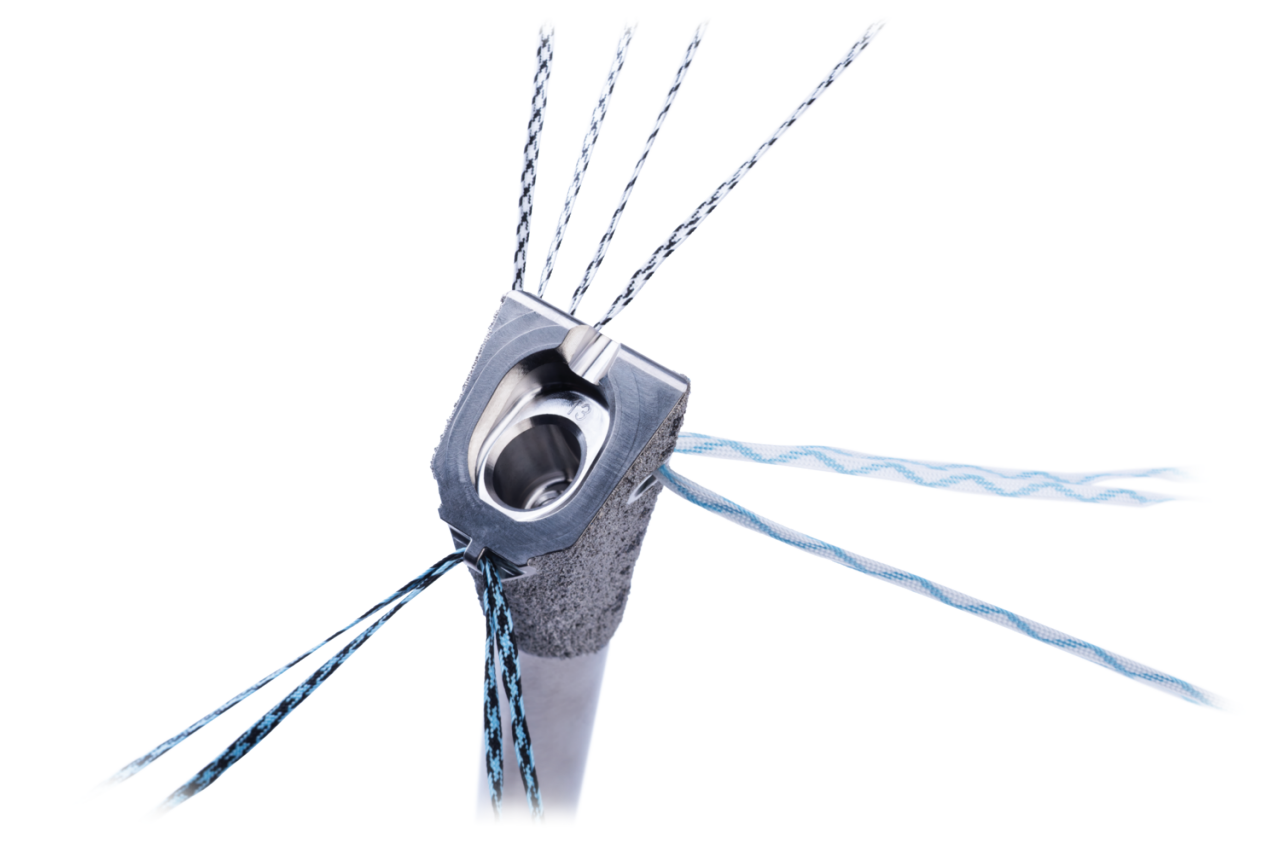
- Identity Revision stems feature three suture holes with one located medially and two more located laterally on both the anterior and posterior sides of the proximal stem

Efficient
- An addition to the Identity Shoulder System, Identity Revision leverages Identity humeral trays, heads and adapters, providing modular optionality to help surgeons meet patient needs in both revision and fracture cases
- The modular nature of Identity Revision gives surgeons several options for lateralization and soft tissue tensioning from both humeral and glenoid sides
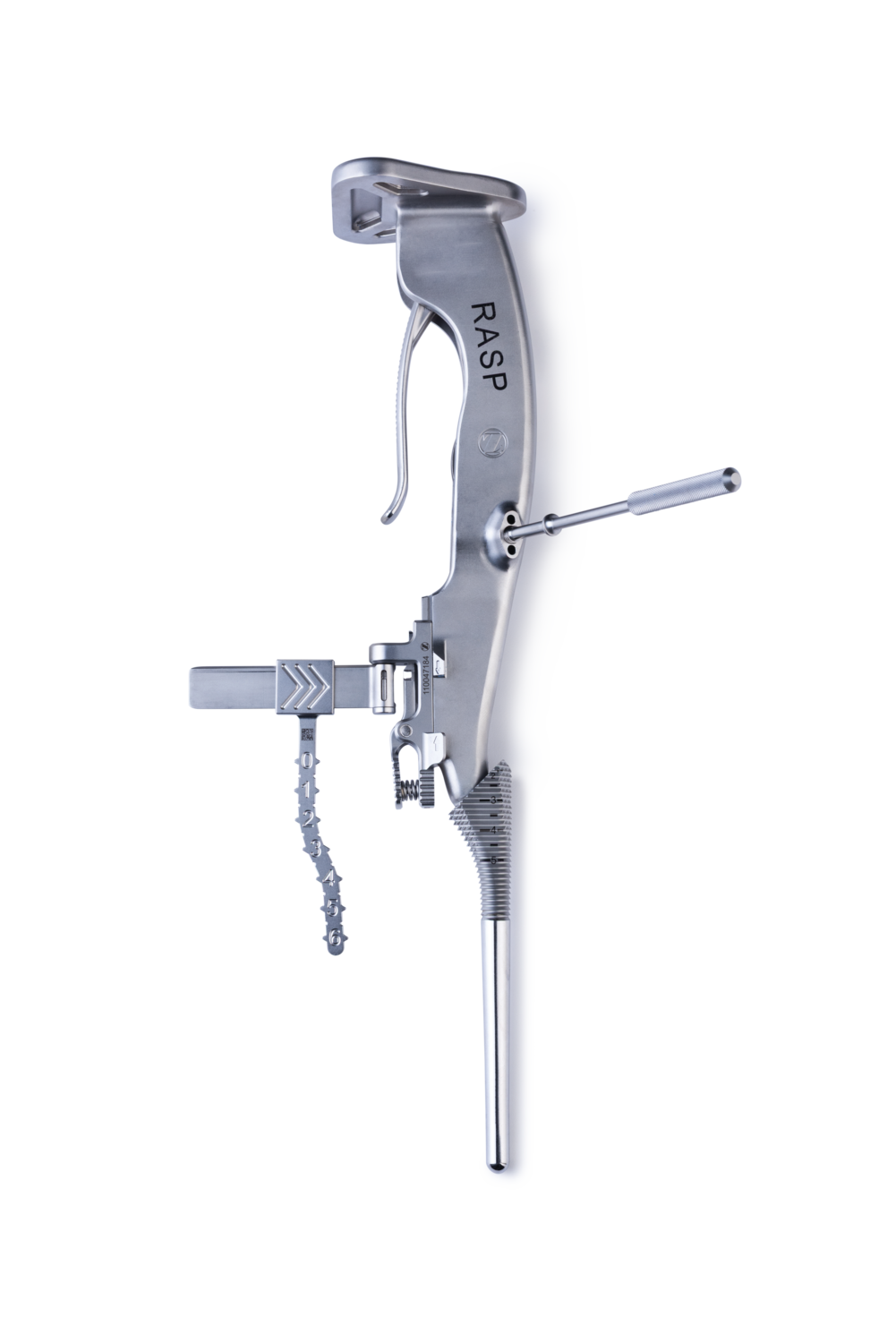
Reproducible
- Identity® Revision instrumentation features numerical etchings, replicated at equal distances across its reamers, rasps and implants to facilitate consistent depth setting from trialing to implantation
- An Identity-specific extramedullary ruler attaches to the reamer, rasp inserter and implant inserter that allows referencing the Pectoralis Major Tendon (PMT) for reproducible canal preparation and humeral height restoration4-6
Videos
Identity Revision Stem Animation Video
Related Products
Additional Information
Sign up to learn more about the Identity Revision Stem
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.