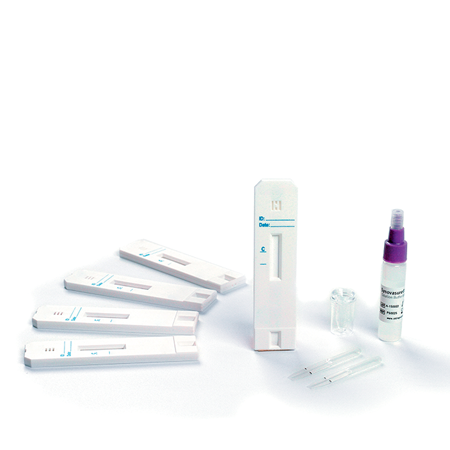
Setting the Stage for Simplified Two-Stage Revision
StageOne™ Cement
Spacer Molds
StageOne technique animation
Overview

StageOne spacer molds are fully customizable and simplify the complexity of a two-stage revision.
StageOne is part of the clinically proven, robust revision portfolio offered by Zimmer Biomet.
Procedures:
- Two-stage Revision Arthroplasty
- Spacer Implantation
Application
- Revision Knee Arthroplasty
Product/service overview:
25%
TKA fail due to PJI2
PJI (Periprosthetic Joint Infection) is one of the most common and devastating complications after total joint replacement; often resulting in prolonged hospitalization and considerable patient morbidity.1 Despite significant technological advancements in implants and techniques, PJI now accounts for 25% and 15% of failed TKA and THA.2 PJI is a significant burden to the overall health care system and is therefore considered one of the largest challenges in orthopedics today3 with increases in cost, longer hospital stay, and a higher likelihood of readmissions compared to primary surgery.4 Our goal is to simplify the complexity of revision care and bring some predictability into the unpredictable.
Benefits
Customizable
StageOne antibiotic bone cement spacers are intended for use in patients undergoing a two-stage revision procedure due to prosthetic joint infection.
The StageOne molds are fully customizable to patient’s needs and part of Zimmer Biomet’s comprehensive portfolio of revision products & services, through one holistic provider.
Simplified Two-Stage Revision
Improved Mobility
Knee Spacer Molds
StageOne Molds are available for the treatment in two stage revision of infected total knee arthroplasty with antibiotic impregnated cement Refobacin® Bone Cement R. The fully customizable molds provide the option for the surgeon to independently address the tibia and the femur with antibiotic bone cement spacers that are available in multiple sizes.
- Designed to retain the patient's natural range of motion
- Maintain soft tissue tension for second stage re-implantation
- Assist in enabling patient ambulation in combination with
- Traditional mobility-assisting devices
- Tibial and femoral sizes are completely interchangeable
- Available in the following sizing options –
- Femur (mm) – 60,65,70,75
- Tibia (mm) – 65,70,75,80
Related Products
Schedule a demo to learn more about fully customizable cement spacer molds for two-stage revision arthroplasty.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.