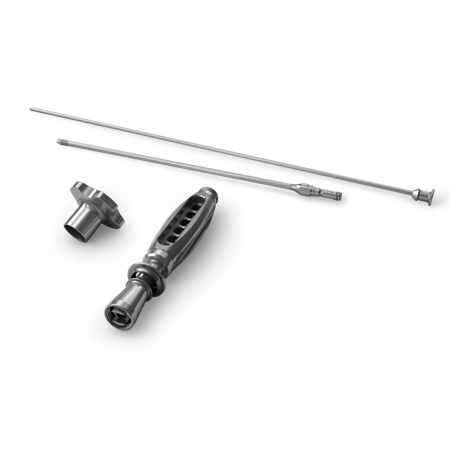
• PerFuse Handle
• PerFuse Strike Cap
• PerFuse Slide Hammer
• PerFuse Slide Hammer Adapter
• Instrument Case
PerFuse Percutaneous Decompression Instrument is designed to access the femoral or humeral head for core decompression. The system is intended to be used for the delivery of bone graft material to an orthopedic site. It facilitates mixing and pre-mixing of bone graft material with I.V. fluids blood, plasma, bone marrow, or other specified blood components deemed necessary by the clinical use requirements.
Core Decompression is a surgical technique to treat avascular necrosis involving drilling one or more channels into the dead bone (necrotic lesion). Creating a channel into the necrotic lesion is intended to relieve intraosseous pressure within the bone and provide a channel to restore blood flow to the diseased bone.1-2
Procedures
Philosophies
Application
The PerFuse system offers flexibility and is easy to use, allowing orthopaedic surgeons to perform a minimally invasive core decompression in multiple applications.


Literature
Watch animation
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.