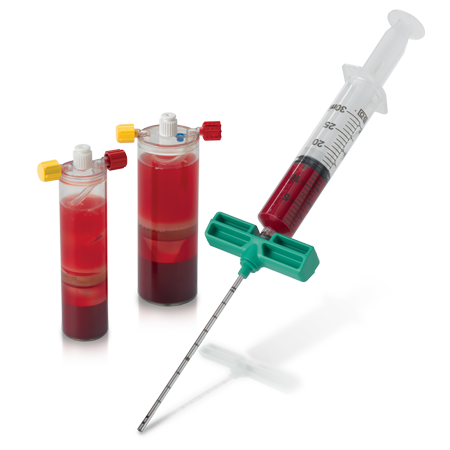
ACD-A Anticoagulant Citrate Dextrose Solution, Solution A
The Only Anticoagulant Product Approved by the United States Food & Drug Administration
ACD-A Anticoagulant Citrate Dextrose Solution, Solution A, USP (2.13% free citrate ion), is a sterile, non-pyrogenic solution. ACD-A is the only anticoagulant product approved by the United States Food & Drug Administration (FDA) for the use in Autologous PRP Systems for the preparation of Platelet-Rich Plasma (PRP).
Procedures
- Multiple
Application
- Autologous PRP
Product Features
Technology
ACD-A Anticoagulant Citrate Dextrose Solution, Solution A, USP (2.13% free citrate ion), is a sterile, non-pyrogenic solution.
Citrate-based anticoagulants prevent the coagulation of blood by virtue of the citrate ion's ability to chelate ionized calcium present in the blood to form a non-ionized calcium-citrate complex.
Benefits
Anticoagulant approved by the FDA
- ACD-A is the only anticoagulant product approved by the United States Food and Drug Administation (FDA) for the use in Autologous PRP Systems for the preparation of Platelet-Rich Plasma (PRP).
Safety Information
Indications
- Anticoagulant Citrate Dextrose Solution, Solution A, U.S.P. (ACD-A) is intended for use as an anticoagulant in the extracorporeal blood processing with Autologous Platelet-Rich Plasma (PRP) Systems in production of PRP.
Warnings and Contraindications
- ACD-A is not for direct intravenous infusion.
Adverse Reactions
- Not applicable. This product is used as an anticoagulant in the extracorporeal blood processing with Autologous PRP Systems in production of platelet rich plasma (PRP).
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.