For any inquiries regarding reimbursement, please contact Zimmer Biomet Reimbursement Hotline: Phone: 1-866-946-0444. Hotline Hours: Monday-Friday, 8am- 5pm, EST
Email: reimbursement@zimmerbiomet.com
Zimmer Biomet’s OCA Kit contains fresh young allograft cartilage and cancellous chips. It is intended to provide surgeons with an early-intervention option to treat osteochondral defects and restore articular cartilage for multiple applications.
Procedures
Philosophies
Application
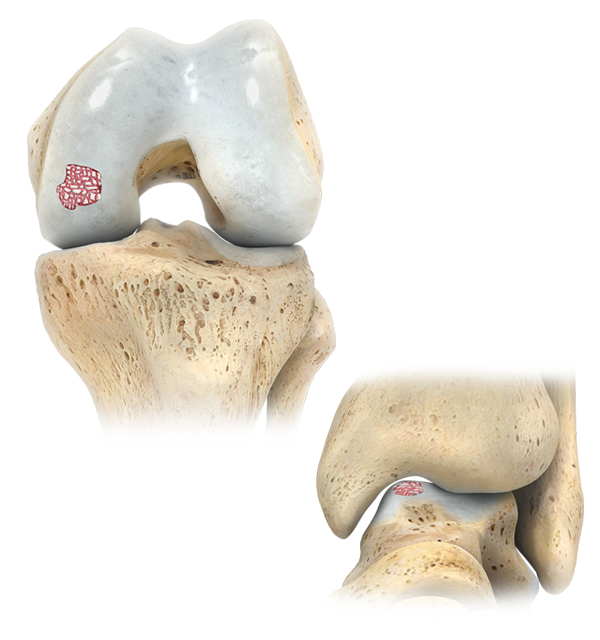

The OCA Kit cartilage graft consists of young living articular cartilage, which contains 10x greater chondrocyte density than adult tissue and does not elicit an allogeneic immune response 4-6*
The modular graft makes it possible to address deficiencies of various sizes and shapes.


All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.