Enhanced Fixation.1 Thoughtful instrumentation.
AFFIXUS® Tibia Nailing System
Designed to deliver enhanced fixation1 with thoughtful instrumentation
The AFFIXUS® Tibia Nailing System is part of a long bone nailing platform designed to treat a range of tibial fractures from simple to complex, with versatile locking options. The AFFIXUS platform is designed to deliver enhanced fixation1 with thoughtful instrumentation for the most common intraoperative approaches.
System Features
Specifications
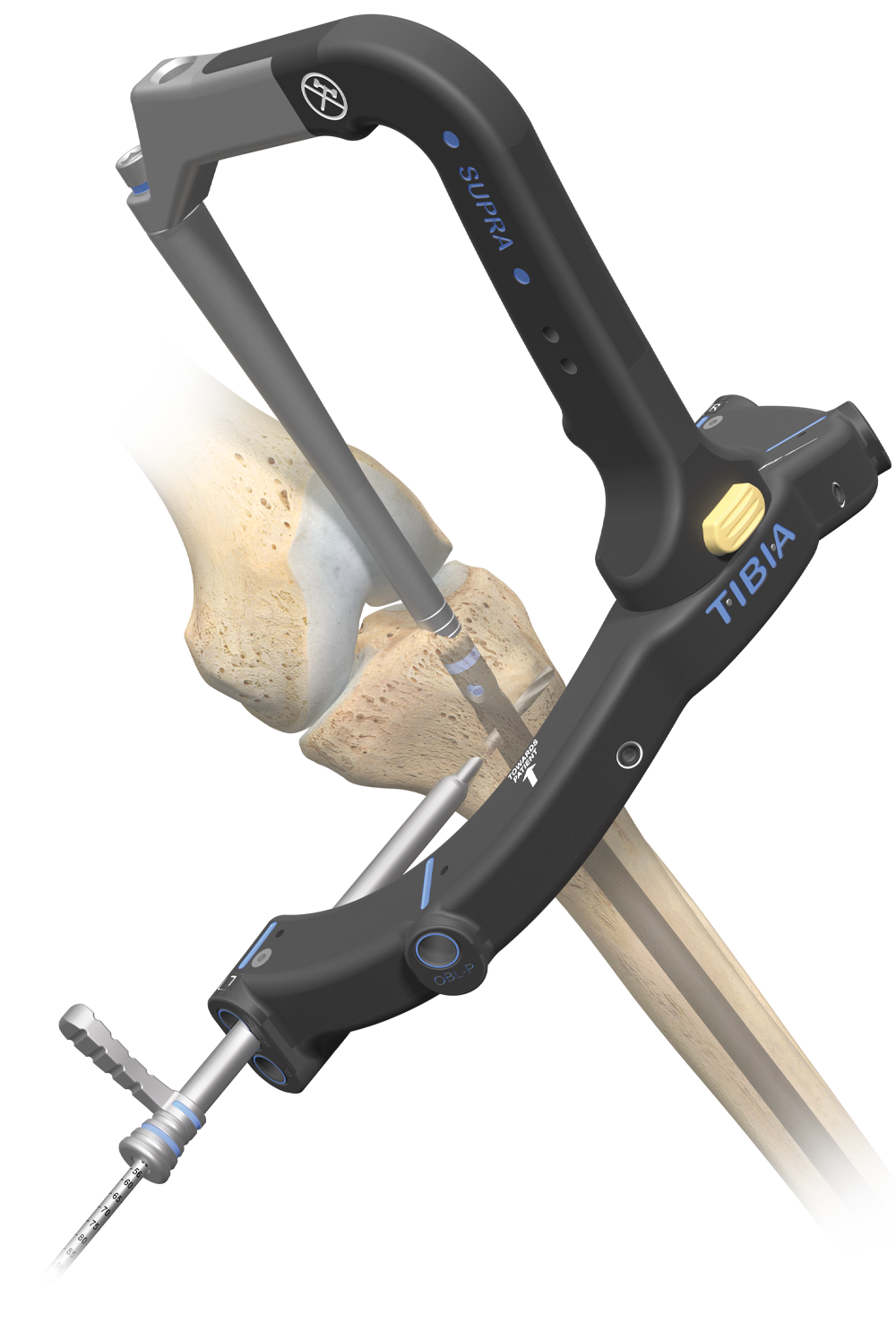
9° Herzog bend is anatomically designed to resist potential proximal fracture displacement
3° distal bend aids in nail insertion
Oblique descending screws allow for capture of distal posterolateral and distal posteromedial fragments
Most distal screw hole is 1.8mm* from the distal end of the nail
*2.4mm for 8mm nail only
Affixus Tibia Nails are identified by a blue band on the proximal end of the nail
Benefits
Enhanced Fixation1
The Affixus Nailing system incorporates our legacy CoreLock™ Technology to mechanically lock the adjacent screws for enhanced fixation, all designed to address the distinct needs of complex fractures.

Thoughtful Instrumentation
Designed with you in mind, this system provides a comprehensive color-coded, hub and spoke solution to simplify instrumentation selection during surgery and reduce shelf space required to support evolving surgeon and patient needs.
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.