Ventix® Link Screw-In Knotless Anchor
Overview
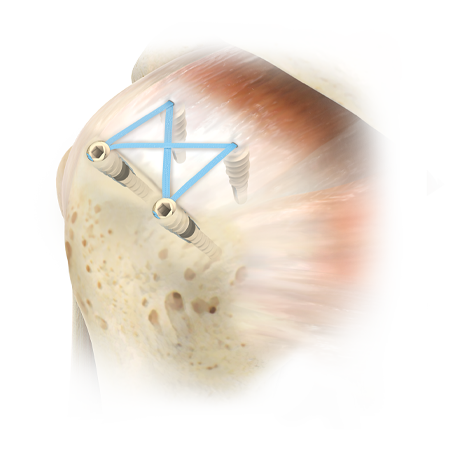
The Ventix Link Screw-In Knotless Anchors bring control, strength and efficiency to soft tissue repair. The threaded, vented, PEEK anchor design provides a strong and reliable platform for lateral row fixation in rotator cuff repair. The unique eyelet/cleat design allows surgeon controlled tensioning after anchor insertion and the ability to accept up to 6 limbs of #2 tape or suture to accommodate all types of tears.
System Features
Screw-In, Knotless, Vented Design
Threaded, vented, PEEK anchor design allows for strong purchase in bone1* and potential bony integration.
The Ventix Link Screw-In Knotless Anchor is designed to address softer bone on the tuberosity
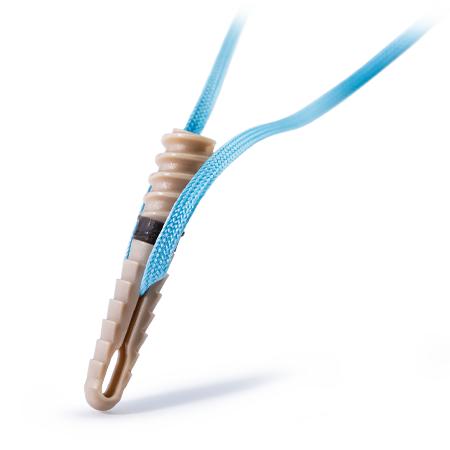
Surgeon-Controlled Tensioning After Anchor Insertion
Unique eyelet/cleat design allows tension to be set, held, and reversed by the surgeon (if needed) after anchor insertion, and maintained throughout deployment.
The Ventix Link Knotless Anchor is designed to address over-tensioning in rotator cuff repair (no need to visualize tension prior to anchor insertion)
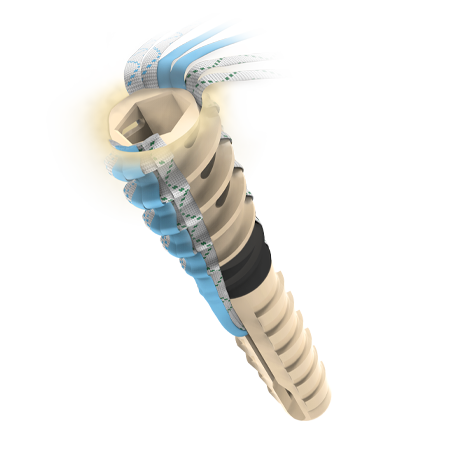
Multiple Suture Fixation Points
Internal cleat mechanism locks suture inside of the anchor during final deployment, in addition to interference fit between the anchor and bone.
Accepts Up to Six (6) Limbs of Suture or Tape:
Ventix Link Knotless Anchors easily accept up to six (6) limbs of #2 BroadBand™ Tape or #2 MaxBraid™ Suture, offering versatility based on the surgeon’s preferred technique.
Videos
Ventix Link Knotless Anchor Animation
Watch animation
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.