Ti-6Al-4V ELI (Type 2 Anodized Titanium Alloy)
Stratum® Ankle
Fusion Plating System
Stratum Ankle Fusion Plating System Animation
A Symphony for Foot and Ankle Repair®
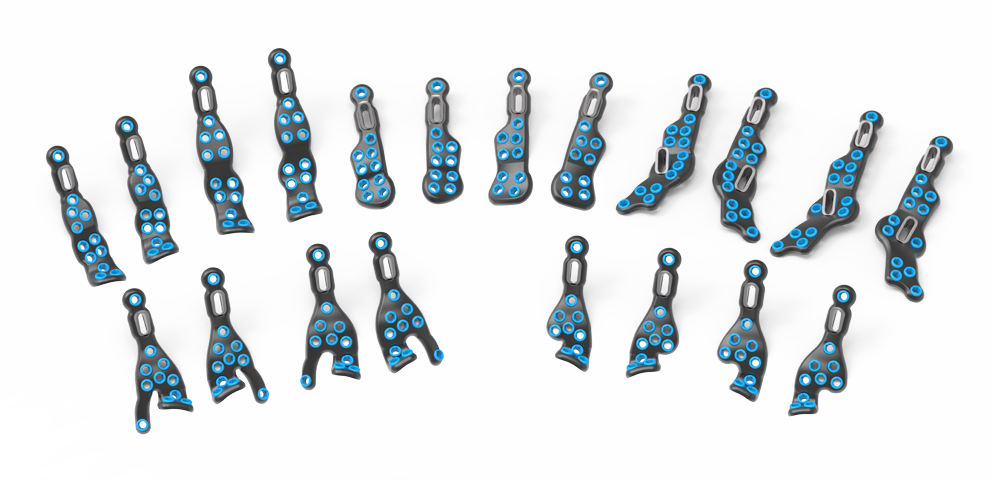
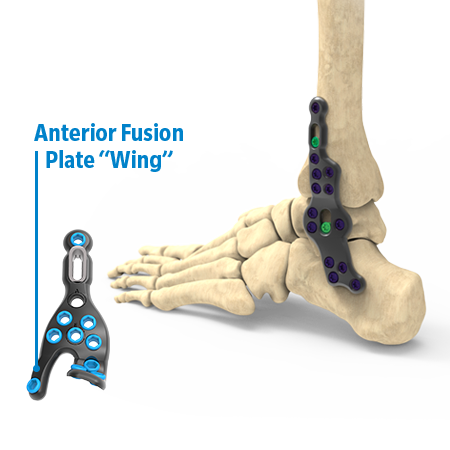
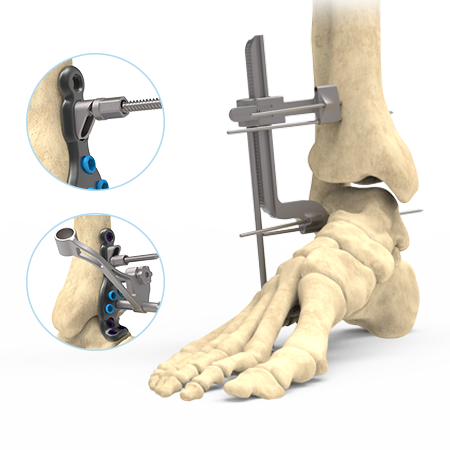
The Stratum Ankle Fusion Plating System offers an anterior, lateral and posterior plating platform, fit to anatomical scans, providing the surgeon with multiple options for anatomical plate application for joint arthrodesis. This system utilizes a joint distractor device designed to provide optimal visualization for joint preparation and patented compression ramps that can achieve up to 7.0mm of linear, bi-cortical compression of the TT & TTC joints. Each plate features an optional outrigger device for guiding a 6.0mm cannulated crossing screw if additional stabilization is desired.
Anatomy
- Ankle
Procedure Type
- Ankle
- Ankle Fusion
System Features
Specifications
Benefits
Efficient
- This system helps address productivity needs for the surgical team by offering an anterior, lateral and posterior plating platform, fit to anatomical scans to provide surgeons with multiple ready to go options for anatomical plate applications.
Low-Profile
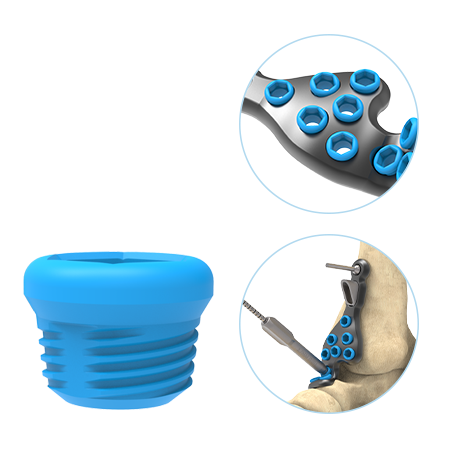
- The anatomically designed plates utilize anatomical scan data to determine optimal plate sizing, paired with low-profile alignment caps with domed edges designed to easily slip under soft tissue.
Versatile
- Plate position can be quickly modified with the ability to bend using insitu-contouring designed to achieve optimal fit to the bone as well as a “wing” option on the anterior fusion plates for additional fixation.
Videos
Stratum® Ankle Fusion Plating System Surgical Animation
Additional Information
Legal Distributor
Zimmer, Inc
1800 West Center St.
Warsaw, IN 46580 U.S.A.
Legal Manufacturer
Medartis, Inc.
1195 Polk Drive
Warsaw, IN 46582, U.S.A.
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.