Featuring Dual Stage CoreLock™ Technology
Phoenix™ Ankle Arthrodesis Nail System
Pre-Assembled Dual-Stage Locking Mechanism
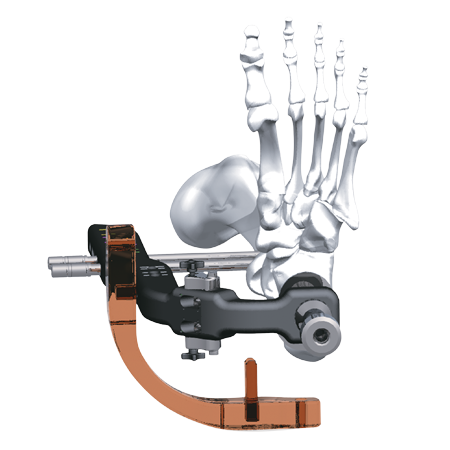
The Phoenix™ Ankle Arthrodesis Nail is composed of titanium alloy and features proprietary CoreLock™ Technology. This distinct, dual-stage locking mechanism is pre-assembled and embedded in each nail, providing up to 7mm of internal tibiotalar compression, followed by independent locking of the calcaneal screws. The nail offers a static screw site proximally and a 10mm dynamic compression slot, both of which accommodate 5.0 mm double lead cortical screws.
Anatomy
- Tibia
Product Type
- Intramedullary Nail
Procedure
- Ankle: Tibiotalocalcaneal (TTC) Arthrodesis
System Features
Specifications
Benefits
Anatomically Designed
- The System is capable of treating varying patient anatomies, the Phoenix Ankle Arthrodesis Nail is universal and available in 10mm, 11mm, and 12mm diameters—all of which are available in lengths of 150mm - 300mm
User-Friendly
- The system features a strong, lightweight radiolucent targeting arm that permits radiographic visualization in multiple planes, and can provide 30mm of axial compression across both the ankle and sub-talar fusion sites. When additional fixation and/or control of rotation is desired, the targeting arm can accurately target an oblique screw on either side of the nail.
Efficient
- With easy to use color-coded instrumentation conveniently contained in a single tray, the Phoenix Ankle Arthrodesis Nail System addresses both patient and surgeon needs
Videos
Phoenix Ankle Arthrodesis Nail System Featuring CoreLock Technology
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.