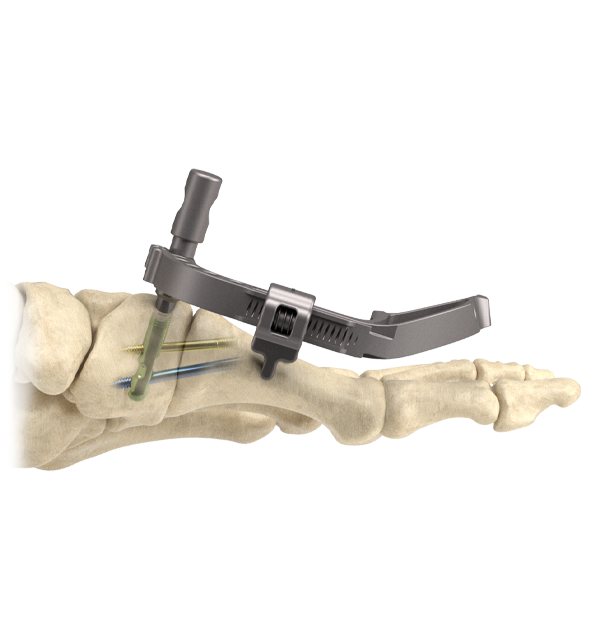
Three-part construct intended for internal fixation for First Tarsometatarsal Fusion.
InCore® Lapidus System
Inspired. Intuitive. InCore.
This system is internal to the bone, and is designed to help minimize the need for hardware removal due to pain and irritation reported with traditional external plating constructs of first tarsometatarsal arthrodesis.1
Anatomy
- Forefoot
- Midfoot
Procedure
- Forefoot: Lapidus
- Midfoot: Lapidus
InCore® Lapidus is designed as a post and screws construct to minimize hardware prominence that can cause pain and lead to hardware removal
Hardware removal due to pain and irritation is reported in up to
17%of first tarsometatarsal arthrodesis cases when using plating constructs.1,2


System Features
Specifications
Benefits
Fully Guided
- Designed to simplify the technique with fully-guided stabilize angular/rotational correction in all three planes. Distraction allows for proper joint visualization and preparation allowing surgeon to cut, curettage or micro fracture. Additionally, the system has a built-in compression/distraction fixture intended to aid and hold pre-compression of the joint.
Solid Intermedullary Construct
- The system includes post and screws designed to minimize hardware prominence that can result in hardware removal due to pain or irritation related to such hardware prominence. Studies have shown up to 17% hardware removal due to pain and irritation when using plating constructs for first tarsometatarsal arthrodesis cases.1,2
Efficient
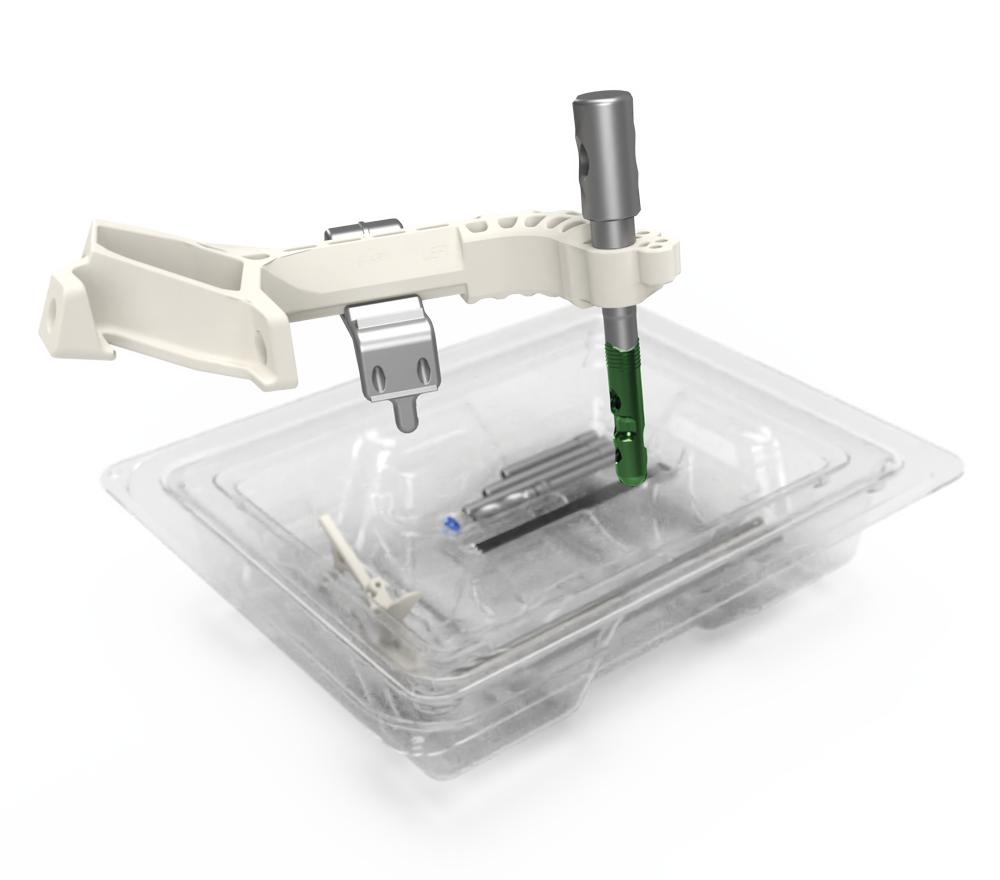
- The easy to use, pre-sterilized kit was intended to decrease time, sterilization cost and contamination concerns. Instruments are new and sharp every time minimizing the complexity for the OR team. Intermedullary implant post comes pre-assembled to the compression/distraction guide, eliminating need for back table assembly.





Education
Literature
Videos
InCore Lapidus System Video
Watch video
InCore Lapidus System Animation
Watch animation
InCore Lapidus System Surgical Demonstration
Watch demonstration
InCore Lapidus System Precision Guided Correction
Watch video
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.