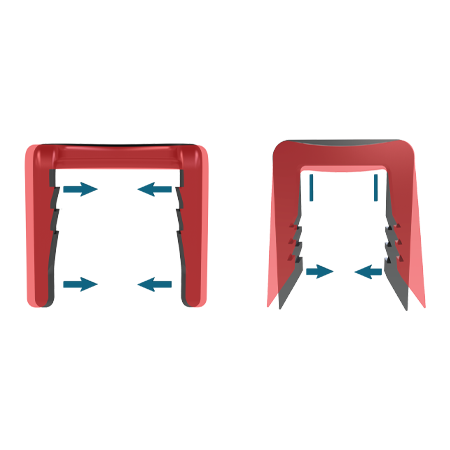
Unique, titanium staple system
ARCUS® Staple System
Arc-Generated Compression
The Arcus Staple System is a unique titanium staple system that utilizes an arc design that creates greater proximal-to-distal compression than tested conventional and nitinol staples1*, internal barbs function to help create compression and to resist migration**
Anatomy
- Forefoot
- Midfoot
- Hindfoot
- Ankle
Procedure Type
- Forefoot: Lapidus, 1st MPJ Arthrodesis, Akin Osteotomy, Fifth Metatarsal Fracture, 1st Interphalangeal Joint Arthrodesis, Hallux Interphalangeal Joint Arthrodesis
- Midfoot: Lapidus, Navicular Fractures, Lisfranc Repair, Naviculocuneiform Arthrodesis, Medial Column Fusion, Talonavicular Arthrodesis, Cuboid Fractures, Cotton Osteotomy
- Hindfoot: Evans Osteotomy, Tibiotalocalcaneal Arthrodesis, Calcaneocuboid, Talonavicular
- Ankle: Tibiotalocalcaneal Arthrodesis
System Features
Specifications
Benefits
Resilient Design
- The titanium is designed to provide balanced elasticity and rigidity.
Clinically Proven
- Proven retention of compression after 600 cycles1. Tooth pattern orientation and shape provide resistance to pullout forces.**
Versatile
- Pre-loaded Staples are available on size specific inserter. Size specific AO drill and drill guide are also included to be fit the right size for anatomy.


Videos
Arcus Staple System-Surgical Animation
Watch animation
Akin Osteotomy with ARCUS® Staple System
Watch surgical demonstration
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.