Designed for Challenging Foot Surgery
A.L.P.S.® Total Foot System
The A.L.P.S. Advantage
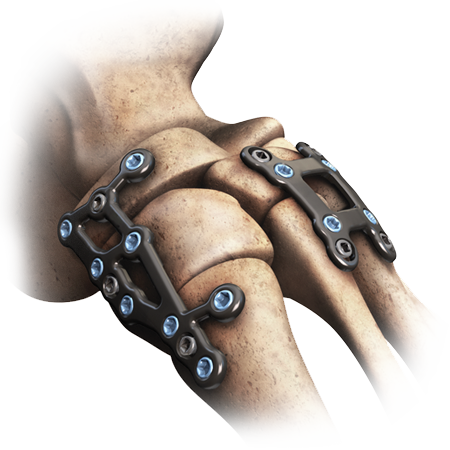
Represents the next generation of anatomic plates specifically designed for the challenges of foot surgery.
Anatomy
- Forefoot
- Midfoot
- Hindfoot
- Ankle
Procedure
- Forefoot: Lapidus, 1st MPJ Arthrodesis, Fifth Metatarsal Fracture
- Midfoot: Lapidus, Navicular Fractures, Lisfranc Repair, Naviculocuneiform Arthrodesis, Medial Column Fusion, Talonavicular Arthrodesis, Cuboid Fractures, Triple Arthrodesis, Cotton Osteotomy
- Hindfoot: Evans Osteotomy, Calcaneocuboid, Talonavicular, Calcaneus Fracture
System Features
Specifications
Benefits
Comprehensive
- The system features a wide variety of plates, screws and instrumentation that have been specifically designed to address both reconstructive and trauma procedures of the forefoot, midfoot and hindfoot.
Flexible
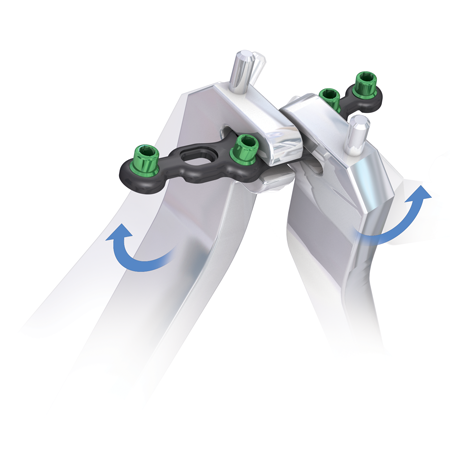
- Flexible plating technology delivers intra-operative customization of the plate and locking, non-locking or multi-directional screw options of varying diameters provide flexibility in constructs.
- The attention to anatomic detail is further enhanced by deliberate regions of flexibility to accommodate individual anatomic variation without compromising strength.
Efficient
- F.A.S.T. Guide Technology facilitates efficient OR time since no intra-operative assembly is required when drilling for locking screws and color coding makes plate identification easy. The result is a comprehensive yet flexible system that is designed to enable OR efficiency and ease of use.




Videos
A.L.P.S. Total Foot System (Animation)
Watch animation
Additional Information
Related Products
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.