Laser Sintered 172 Mpa4
One2One™ HTR-PEKK
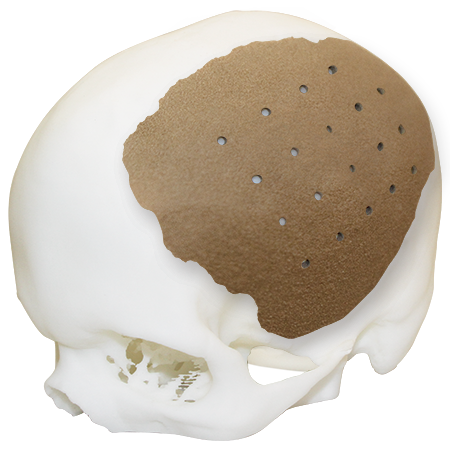
Patient-Matched Cranial Implant

Overview
One2One HTR-PEKK Patient-Matched Implants are created through state-of-the-art technology and a novel material, PEKK (poly-ether-ketone-ketone) for manufacturing implants. Complex cranial cases require a solution that offers the patient the best anatomical fit and proven biocompatibility.1
Procedures
- Cranial Reconstruction
Application
- Secondary
Product Features
Specifications
Benefits
High Mechanical Strength Material
- Compressive strength of 172 Mpa4
Biocompatible1
- PEKK material is from same polymer family as PEEK which has been utilized in Orthopedics and Trauma since the 1980's.6
Patient-Specific Anatomic Fit
- Designed and manufactured based on uncompressed DICOM (Digital Imaging and Communications in Medicine) CT
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.