Nextra CH Cannulated Hammertoe System is comprised of cannulated implants and instruments to provide targeting guidance for reproducible procedures.
Nextra® CH Cannulated Hammertoe Correction System
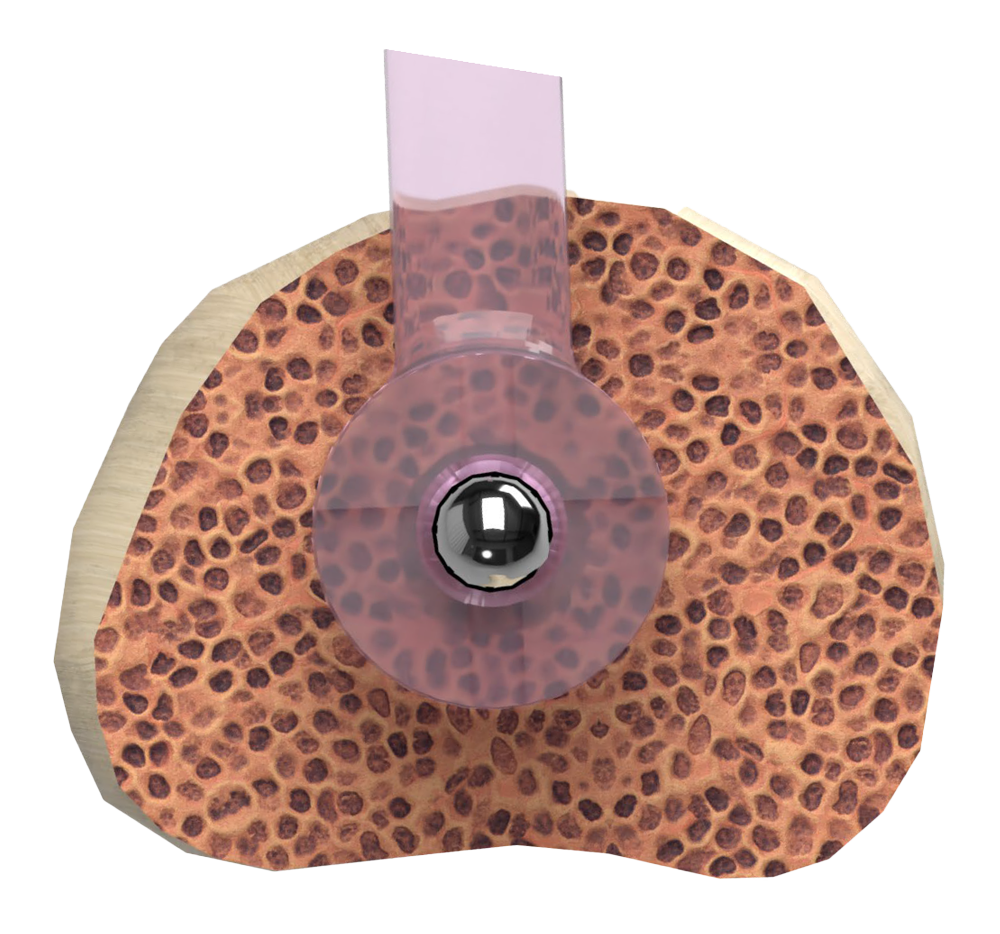
Two-Piece Design
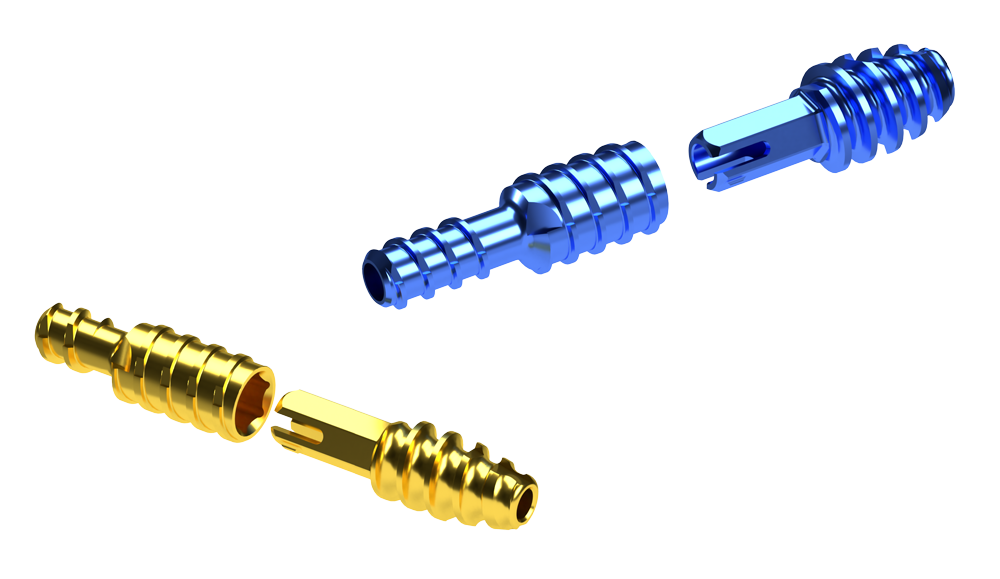
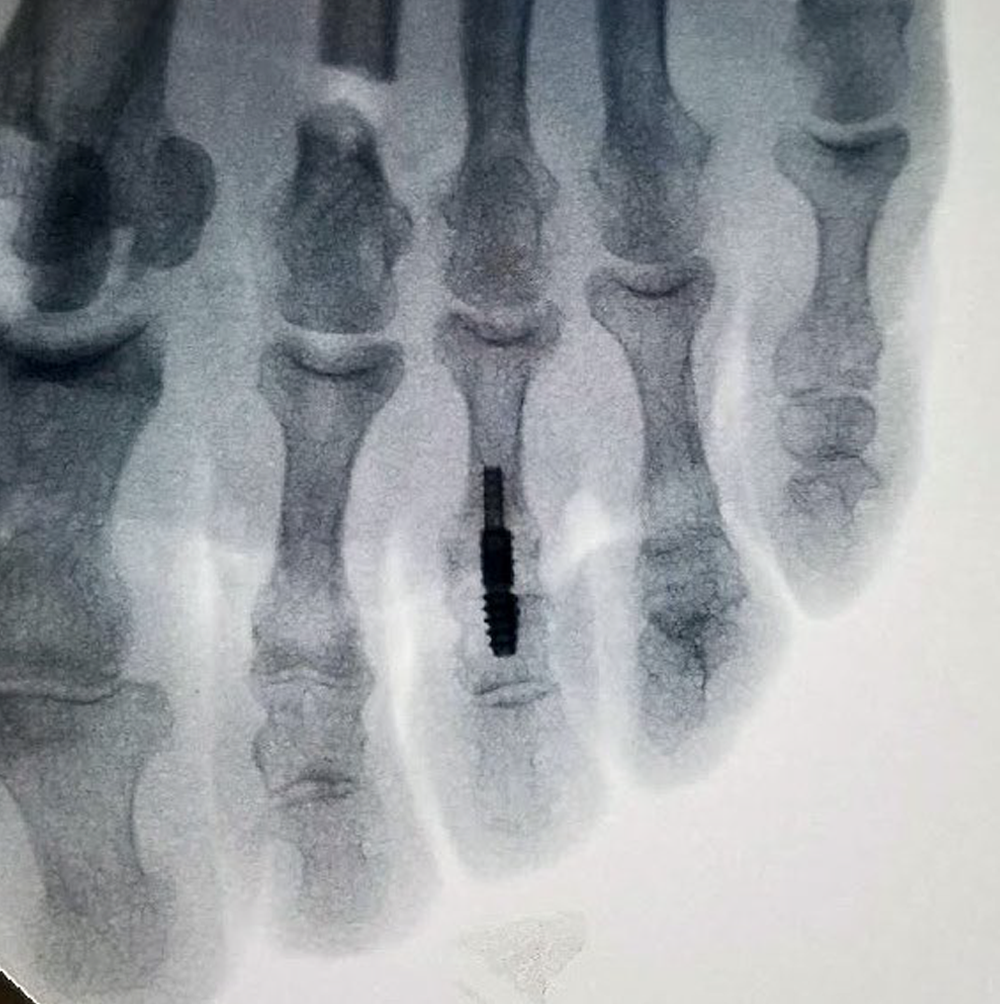
The two-piece cannulated design allows for optional technique to pin metatarsophalangeal joint
- Two-piece threaded implant construct designed for optimal bone purchase
- Cannulated implants and instruments provide targeting and technique guidance for repeatable procedures
- Implant-to-implant rotational stability via differentiated hexagonal locking design
- Variable implant locking position provides in-situ adjustability before final closure
- Allows for optional technique to pin metatarsophalangeal joint
- Single-use sterile packed kit
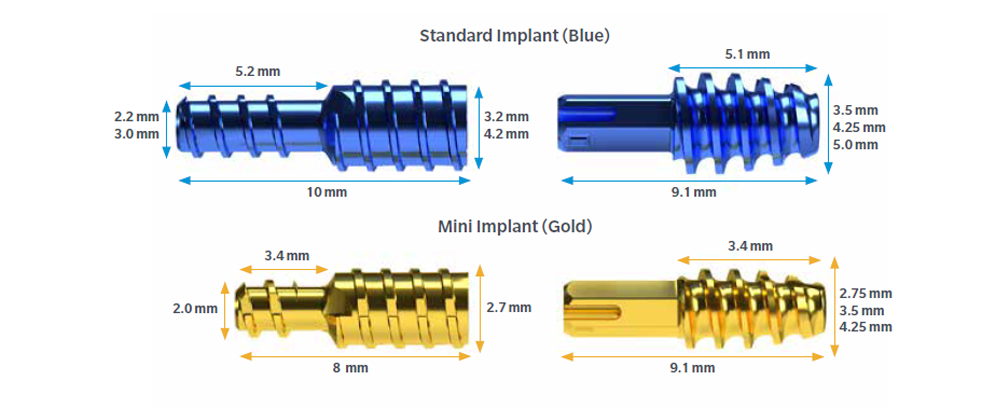
Specifications
Standard
- 3.2mm and 4.2mm proximal diameter
- 3.5mm, 4.25mm, 5.0mm diameters in middle phalynx
Mini
- 2.7mm proximal diameter
- 2.75mm, 3.5mm, and 4.25mm diameters in middle phalynx
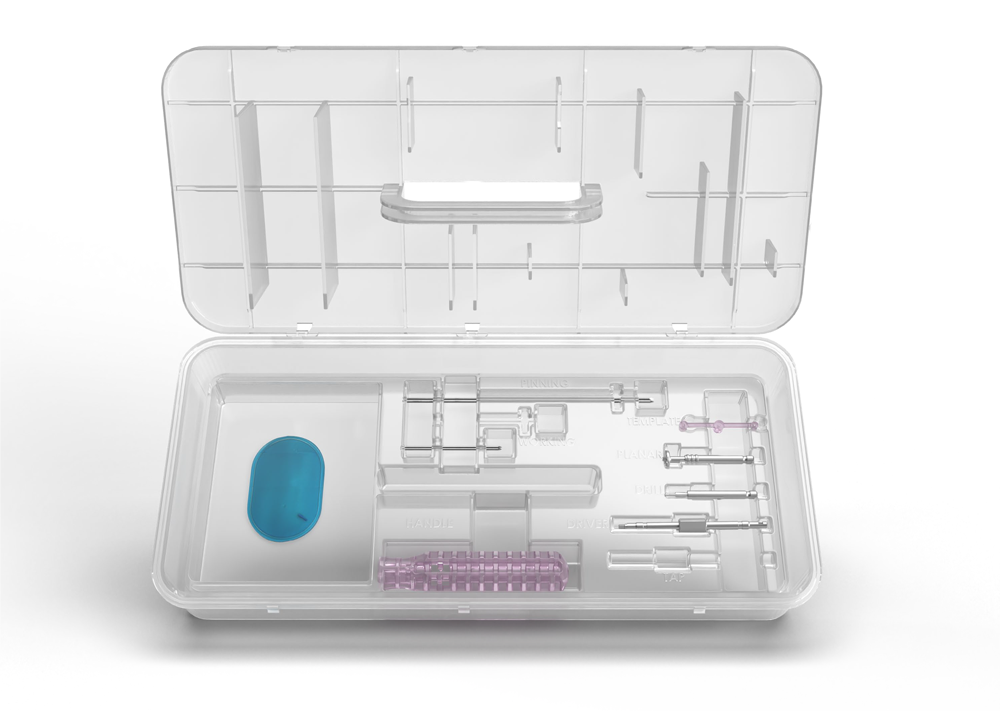
- Single-use instrument tray
- Designed for precise, reproducible procedures
- Optimized for OR efficiency
- Sizing guide for canal diameter
Videos
Nextra® CH Cannulated Hammertoe System - Surgical Animation
Watch animation
Hammertoe Correction - Nextra® CH System - Surgical Demonstration
Watch video
Additional Information
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
Zimmer Biomet and Nextremity Solutions do not practice medicine. This technique was developed in conjunction with health care professionals. This document is intended for surgeons and is not intended for laypersons. Each surgeon should exercise his or her own independent judgment in the diagnosis and treatment of an individual patient, and this information does not purport to replace the comprehensive training surgeons have received.
As with all surgical procedures, the technique used in each case will depend on the surgeon’s medical judgment as the best treatment for each patient.
Results will vary based on health, weight, activity and other variables. Not all patients are candidates for this product and/or procedure. Caution: Federal (USA) law restricts this device to sale by or on the order of a surgeon. Rx only.
Nextremity Solutions is a trademark of Nextremity Solutions, Inc.
Zimmer Biomet is the exclusive distributor of the Nextra® CH Cannulated Hammertoe System
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.