Fully Guided, Solid Intraosseous Construct
InCore Subtalar
Overview
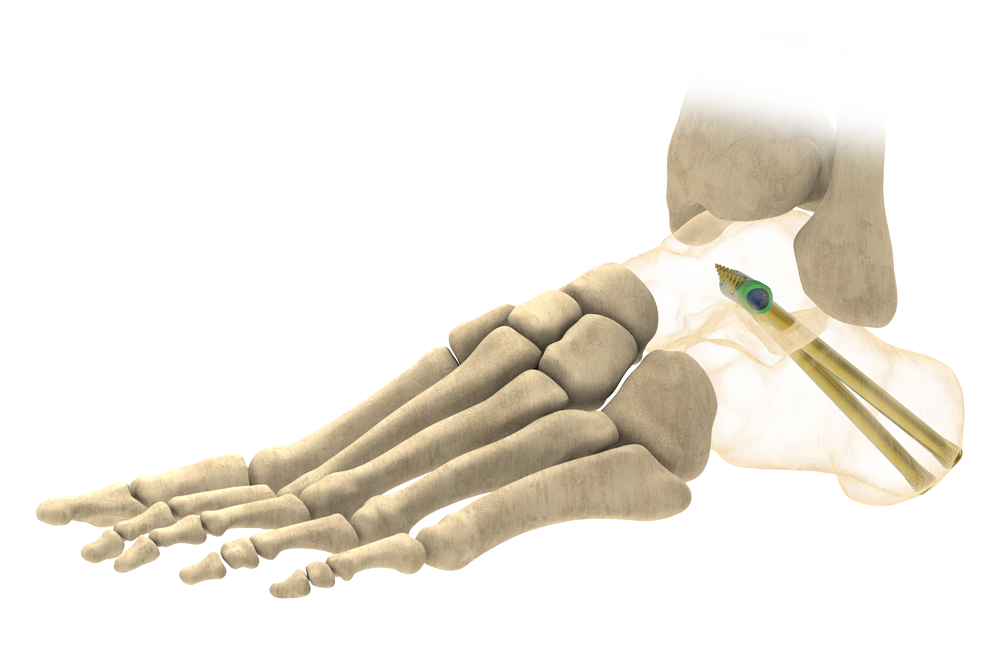
The InCore Subtalar system is a fully guided, solid intraosseous construct designed to minimize painful hardware prominence, while providing mechanical compression at the subtalar joint.
System Features
Fully Guided
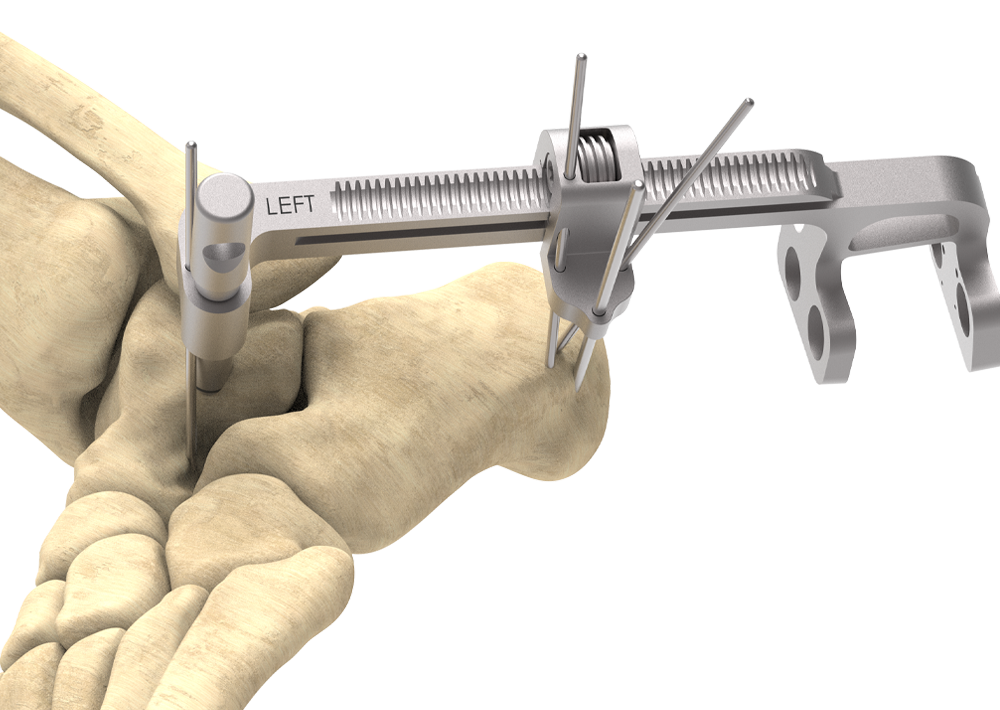
- Post and Targeting Guide utilize anatomical landmarks to facilitate fixation placement
Solid Intraosseous Construct
- Solid 8.0mm Titanium Post provides large surface area engagement in the cancellous bone of the talus
- Headless compression screws thread directly into the 8.0mm post
- The post and screws construct is designed to fit in the bone to help minimize painful hardware prominence often reported with headed screws1,2*
Controlled Distraction/Compression
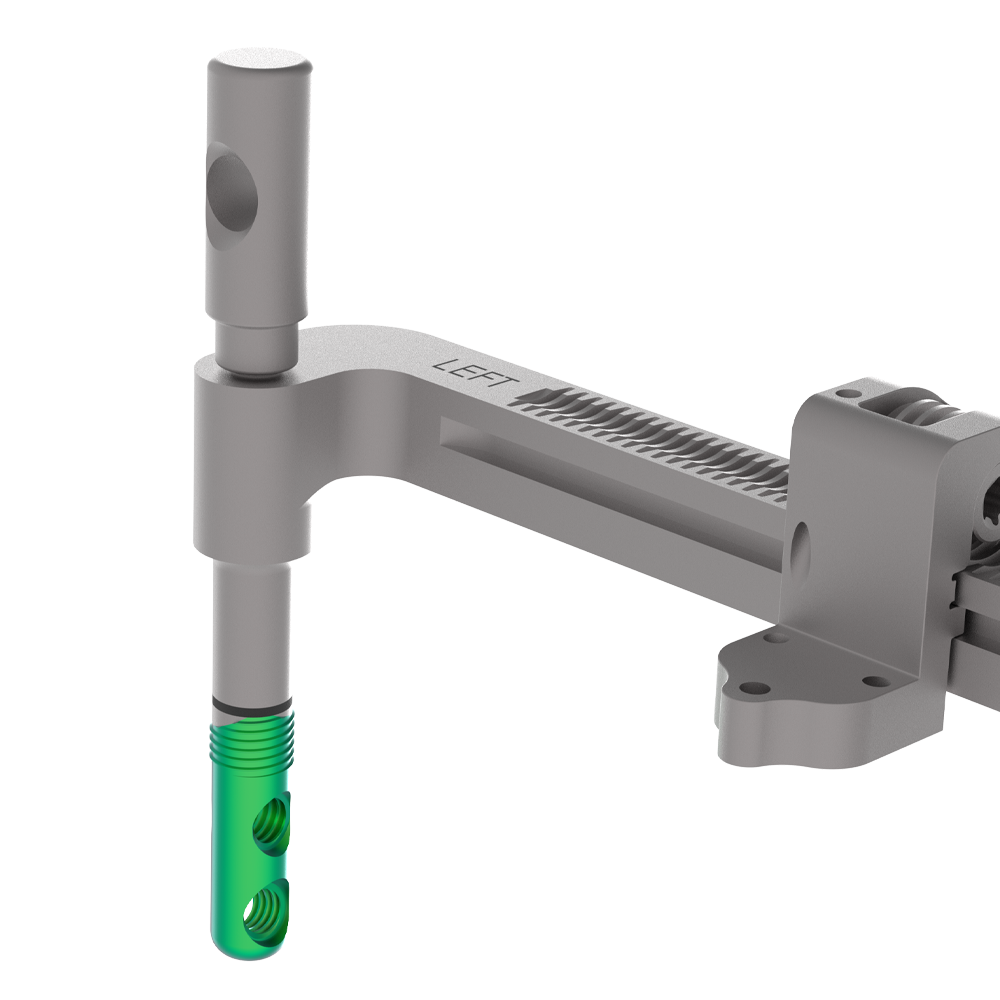
- Targeting Guide includes built-in Compression-Distraction Fixture providing compression parallel to the long axis of the screws
- Targeting Guide provides distraction of the joint for visualization and joint preparation
- Distraction allows space for curettage and microfracture
Specifications
- 8.0mm x 28mm Titanium Post
- 5.5mm Diameter Headless Compression Screws offered from 60mm to 110mm in length
- T25 Hexalobe Driver
Videos
InCore Subtalar System - Surgical Animation
Watch animation
InCore Intraosseous Animation
Watch animation
InCore Subtalar Cadaveric Demonstration
Watch animation
Additional Information
*InCore Subtalar has not been comparatively clinically evaluated specifically for hardware-related pain
Tailored resources for your patients.
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.
All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the instructions for use or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.