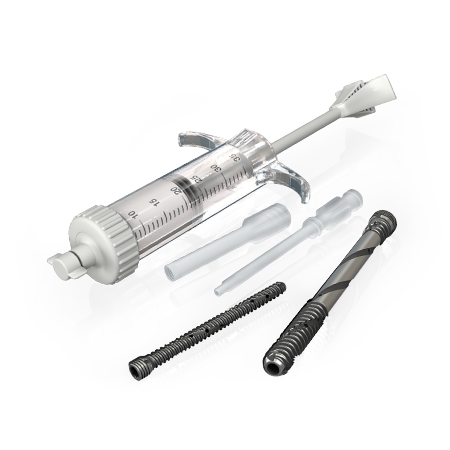
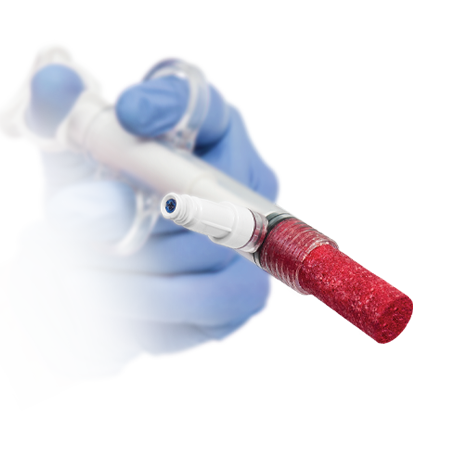
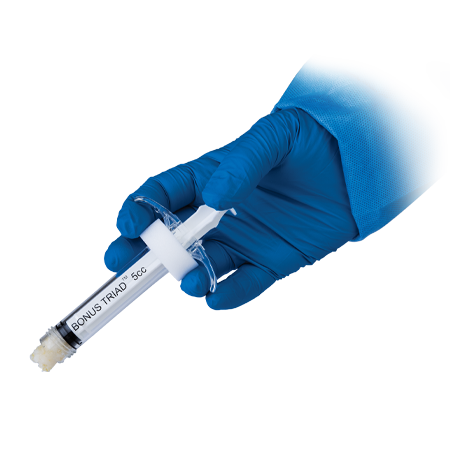
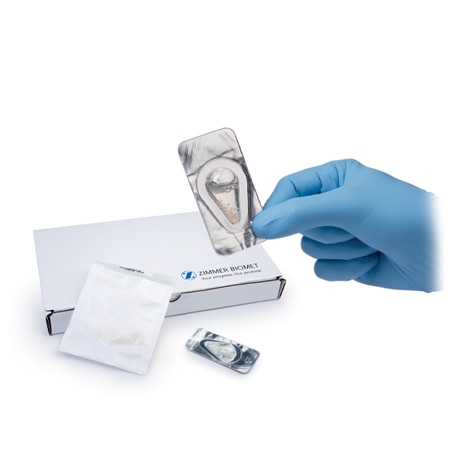
One of the most comprehensive Biologics portfolios within the market today.
Biologics
The Zimmer Biomet Biologics product portfolio represents the passionate pursuit of the most innovative and clinically relevant solutions addressing the needs of surgeons and their patients. As a leader in autologous therapies, Zimmer Biomet Biologics offers a myriad of solutions for hard tissue applications, but also for soft tissue management.
Autologous Therapies
Autologous cellular therapies are at the forefront of regenerative medicine. Such treatment options include blood and bone marrow aspirate and concentration.
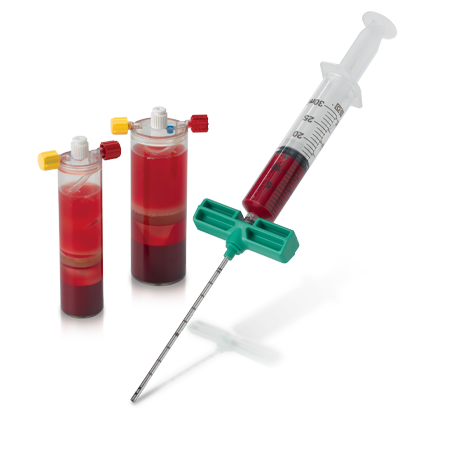
Zimmer Biomet offers Platelet-Rich Plasma (PRP) products to produce PRP which can be mixed with autograft or allograft materials.
Ancillary Products
Zimmer Biomet offers a host of ancillary products which help support and manage the delivery of our extensive product portfolio.
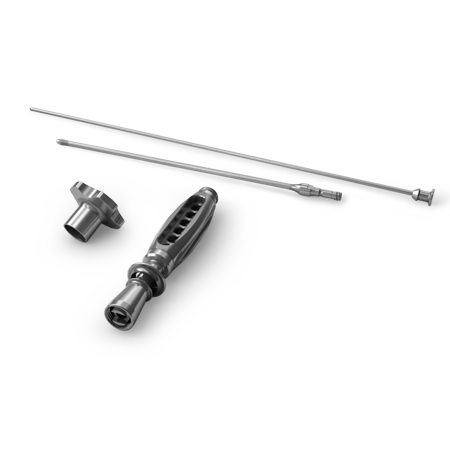
Bone Grafting
The philosophy defining our hard tissue products is based on the
complex process involved in tissue repair, wherein the matrix/scaffold
(osteoconductive), signaling proteins (osteopromotive* and/or
osteoinductive), and tissue forming cells (osteogenic) work in concert
to form new tissue (bone).3
Although autograft is considered the gold standard for
orthopedic procedures requiring graft material, it has known
limitations with associated donor site morbidity and limited
availability.4 Synthetic bone graft substitutes have been
developed to overcome these limitations of donor site morbidity and
limited availability.
* Products that produce a PRP output do not currently have FDA clearance to be characterized with a specific mechanism of action. PRP, in and of itself (i.e., without the autograft or allograft), is not FDA cleared as “osteopromotive.”
Viable Cellular Matrix
Zimmer Biomet offers human tissue allograft possessing all three of the components of the bone remodeling triad: osteoconductivity, osteoinductivity and osteogenicity.
Cartilage Repair
The cartilage repair solutions from Zimmer Biomet can be used to address osteochondral deficiencies in multiple anatomic locations. They offer a convenient early interventions option that can limit the need for further surgical intervention and potentially divert the progression of these lesions.
Soft Tissue Management
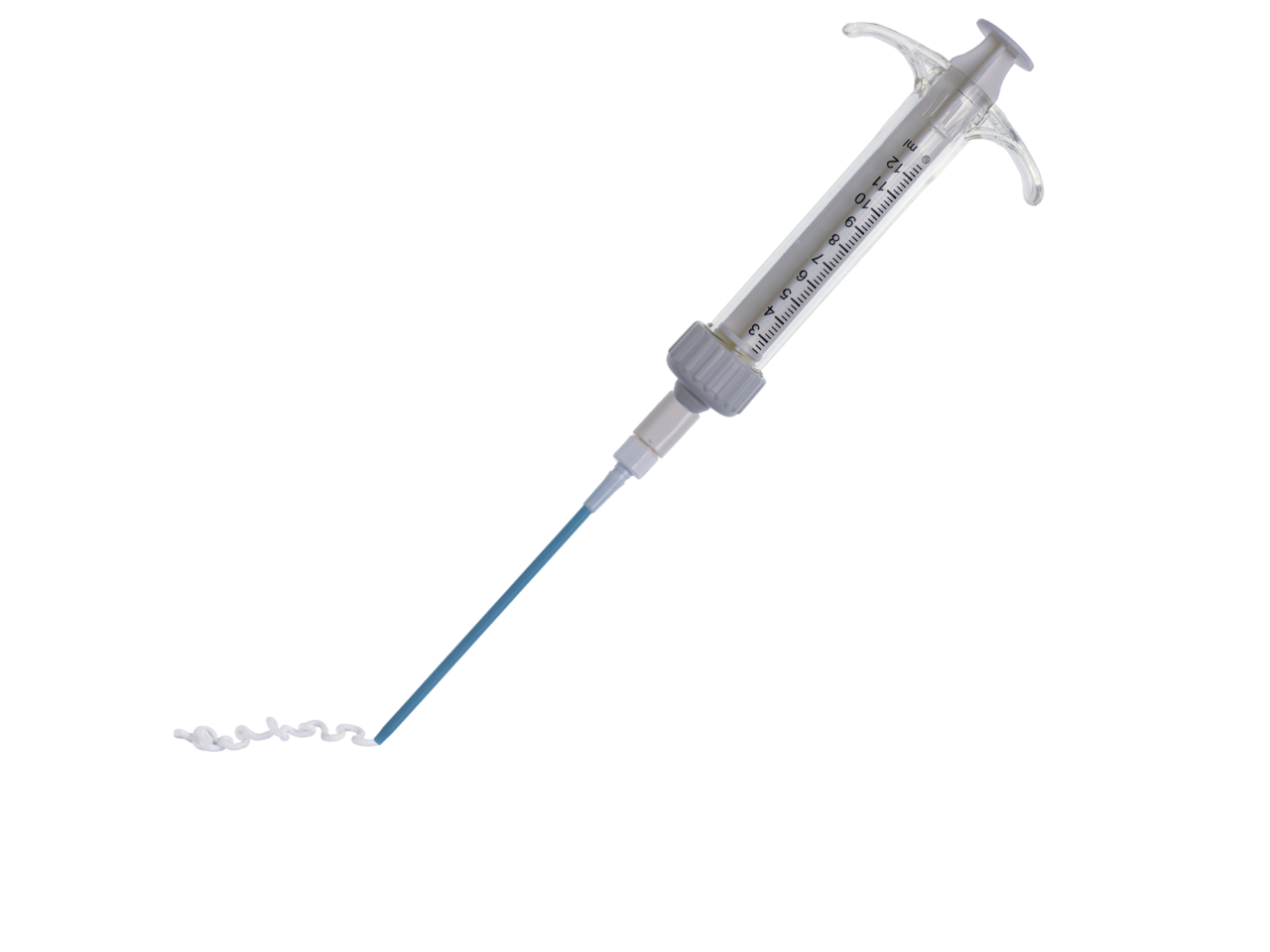
Zimmer Biomet offers soft tissue management products which may be used in various practices including orthopedics, plastic surgery and general surgery.
Tailored resources for your patients
Find videos, articles, and interactive content to guide your patients throughout their surgical journey on ReadyPatient.com, our dedicated patient recovery site.

All content herein is protected by copyright, trademarks and other intellectual property rights, as applicable, owned by or licensed to Zimmer Biomet or its affiliates unless otherwise indicated, and must not be redistributed, duplicated or disclosed, in whole or in part, without the express written consent of Zimmer Biomet.
This material is intended for health care professionals. Distribution to any other recipient is prohibited.
For product information, including indications, contraindications, warnings, precautions, potential adverse effects and patient counseling information, see the package insert or contact your local representative; search this website for additional product information. To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please visit labeling.zimmerbiomet.com or call 1-800-348-2759, press 4 for 411 Technical Support.